Abstract
Lactate/Pyruvate ratio (LPR) from microdialysis is a well established marker of cerebral metabolic crisis. For brain injury patients, abnormally high LPR could indicate cerebral ischemia or failure of O2 uptake. However, there is a debate on what is primary factor responsible for LPR increase. Exploiting the potential of using the morphology of a high temporal resolution signal such as intracranial pulse (ICP) to characterize cerebrovascular changes, a data analysis experiment is taken to test whether consistent changes in ICP pulse morphological metrics accompany the LPR increase. We studied 3517 hours of LPR and continuous ICP data from 19 severe traumatic brain injury (TBI) patients. Our Morphological Clustering and Analysis of Intracranial Pressure (MOCAIP) algorithm was applied to ICP pulses, which were matched in time to the LPR measurements, and 128 pulse morphological metrics were extracted. We automatically identified the episodes of LPR increases using a moving time-window of 10 to 20 hours. We then studied the trending patterns of each of the 128 ICP MOCAIP metrics within these identified periods and determined them to be one of the following three types: increasing, decreasing, or no-trend. A binomial test was employed to investigate whether any MOCAIP metrics shows a consistent type of trend among all episodes of LPR increase per patient. Regardless of the selected values for different parameters of the proposed method, for the majority of the subjects in the study (78%), none of the ICP metrics show any consistent trend during the episodes of LPR increase. Even for the few subjects who have at least one ICP metric with a consistent trend during the LPR increase episodes, the number of such metrics is small and varies from subject to subject. Given the fact that ICP pulse morphology is influenced by the cerebral vasculature, our results suggest that a dominant cerebral vascular cause may be behind the changes in LPR when LPR trends correlate with ICP pulse morphological changes. However, incidence of such correlation seems to be low.
Keywords: Intracranial pressure, Hemodynamic signal, Microdialysis, Lactate/Pyruvate ratio, Waveform morphology
1-Introduction
Intracerebral microdialysis (MD) is a brain monitoring modality introduced recently for neurocritical care of brain injury patients. The chronic implementation of MD probes in the human brains was first studied by Persson et al. in 1992(Persson and Hillered, 1992). The authors found a 25-fold increase in extracellular fluid glutamate, aspartate, and taurine under conditions of energy perturbation in patients in the neurosurgical intensive care unit, as indicated by high levels of the lactate/pyruvate ratio (LPR). Since then, there have been several studies to examine the patterns of change in microdialysate concentrations of glucose and LPR in acute brain injury conditions. Hlatky et al. showed that although changes in the brain tissue PbtO2 provided the earliest sign of hypoxia/ischemia, the microdialysis assays provided additional information about the consequences that the reduced tissue PbtO2 has on brain metabolism and this information may be helpful in managing the critically ill patients (Hlatky et al., 2004). Vespa et al. compared microdialysis values with the regional mean positron emission tomography values adjacent to the probe and concluded that traumatic brain injury leads to a state of persistent metabolic crisis as reflected by abnormal cerebral microdialysis LPR that is not related to ischemia(Vespa et al., 2005). More recently, the authors in (Timofeev et al., 2011b) reported that perilesional tissue chemistry has demonstrated a significant independent relationship with ICP, PbtO2 and cerebral perfusion pressure (CPP) thresholds, with increasing LPR in response to decrease in PbtO2 and CPP, and increase in ICP. In another work from the same group (Timofeev et al., 2011a), it was shown that LPR and mean ICP; among other parameters e.g. cerebrovascular pressure reactivity index, were significantly higher in patients who had died comparing to the ones who survived.
The common theme in these existing studies has been to correlate MD metrics such as LPR with variables obtained from other brain monitoring or imaging tools to gain better understanding of both measurements. Given the fact that these measurements arise from complex hemodynamic and biochemical processes, such a cross-modality approach is very valid in promoting a deeper analysis to gain a more comprehensive clinical picture that these measurements project. The present work furthers our previous studies of using ICP pulse morphology as a probe of cerebrovascular changes (Asgari et al., 2011) to test the hypothesis that there will be consistent ICP morphological changes in correspondence to the episodes of LPR increase if cerebral vasoconstriction is the primary reason for LPR increase.
ICP pulses have a clear vascular origin as demonstrated in the experiments reported in some early studies (Adolph et al., 1967; Laitinen, 1968). As such, cerebrovascular changes, especially those of acute nature, can modulate the shape of individual ICP pulses. Such observations are abundant in existing reports (Cardoso et al., 1983; Cardoso et al., 1988; Czosnyka et al., 1996; Solheim et al., 2008). However, a more quantitative approach to characterize this has only been possible recently due to the availability of new tools of analyzing ICP pulse morphology (Eide, 2006; Ellis et al., 2007; Hu et al., 2009). In particular, we studied ICP pulse morphological changes, across different patients, caused by hypercapnia-induced acute cerebral vasodilatation (Asgari et al., 2011). Applying Morphological Clustering and Analysis of Intracranial Pressure (MOCAIP) algorithm to each ICP pulse, 128 morphological metrics were extracted and the consistency and rate of change for each individual metric was assessed. The results demonstrated that the acute cerebral vasodilatation/vasoconstriction causes consistent changes in 78 out of these 128 ICP MOCAIP metrics. Built upon this previous study, we aim to use the MOCAIP analysis of ICP to investigate whether consistent morphological changes of ICP pulse accompany LPR increases. This investigation would hence help test our hypothesis because consistent alternations of ICP pulse morphology would be a necessary condition for the presence of a mono-phasic cerebrovascular changes.
2- Materials and Methods
2.1. MOCAIP algorithm
MOCAIP algorithm has been recently developed for the automatic extraction of morphological features of ICP pulses in real time (Hu et al., 2009). The framework is capable of enhancing ICP signal quality, recognition of legitimate (not contaminated with noise and artifacts) ICP pulses and detection of the three sub-peaks and three sub-nadirs of an ICP pulse. Following the identification of these six landmarks on an individual ICP pulse, MOCAIP extracts 128 additional pulse morphological metrics based on the identified landmarks. Thus far, the proposed algorithm has been validated as a technical advancement toward a new paradigm of more comprehensive information extraction from ICP in several studies; prediction of acute ICP elevation(Hu et al., 2010), continuous detection of cerebral hypoperfusion(Hu et al.), automated classification of ICP B-waves (Kasprowicz et al.) and detection of cerebrovascuar changes happening during vasodilation/vasoconstriction(Asgari et al., 2011). Also the performance of different individual processing blocks of the algorithm has been significantly improved applying novel and sophisticated signal processing method(Asgari et al., 2009; Asgari et al., 2010; Scalzo et al., 2009; Scalzo et al., 2010).
The readers are referred to(Asgari et al., 2011) for more information regarding the identification of the six landmarks on an individual ICP pulse and extracting 128 additional pulse morphological metrics.
2.2. Automatic Detection of Lactate/Pyruvate ratio (LPR) Increase Episodes
In order to perform automatic identification of the episodes of the LPR increase, the following procedure was implemented for each patient. A moving window of length L samples with η % overlapping were applied to the collected LPR data. By applying a constraint on the time length of each resulted subsegment (τ in hours) as T 2 < τ < T, the unreasonably long (or unreasonably short) subsegments were excluded from further processing. Then the LPR data of the subsegments with reasonable time length were resampled at a sampling frequency of 1 sample/hour and a line was fitted to the resampled data using an iteratively reweighted least square with a bisquare weighting function(Holland and Welsch, 1977). This robust regression technique (Hadi and Simonoff) minimizes the influence of poor quality LPR data points on the goodness of the fit and provides more precise estimates of the parameters of the model ( b = [slope, intercept]T ). Therefore, additional scale factors (weights) that are inversely proportional to the variance at each level of LPR data were included in the least square fitting process as Minimize ([Y – Ŷ]T ×W ×[Y – Ŷ]), where Y and Ŷ are the vectors of N observed and estimated LPR data points over each subsegment, respectively. W is the diagonal weight matrix whose elements are given as
and ui, i 1,…, N are the adjusted and standardized residuals(Hadi and Simonoff, 1993). Denoting X as the vector of time data points for each subsegment, the parameters of the model were estimated as b = (XT WX )−1 XT WY and two diagnostics statistics for the regression were calculated; p the probability of t-statistic (the coefficients divided by their standard error) and wmin the minimum weight among the diagonal elements of matrix W.
An LPR increase episode was defined as a sub-segment with p ≤ thres p and wmin ≥ thresw where thresp and thresw are two thresholds to adjust the degree of the goodness of the fit and the sensitivity to the outliers, respectively. In the present work, the baseline values for parameters that determine the episodes of LPR increase are set as: L = 10 samples, η = 90%, T = 20 hours, thres p = 30th percentile of all p values for the corresponding patient, and thres w = 95th of the wmin values for the corresponding patient.
2.3. Determining the Trend of Change of the ICP Metrics during the Identified Episodes of LPR Increase
Following identification of the LPR increase episodes for each patient, we determine the direction and rate of change for each of 128 ICP MOCAIP metrics by adopting the same line fitting procedure for LPR, as described in the last section, to the values of the metric over the corresponding LPR increase episode. The values of ICP metrics for a specific LPR increase episode are obtained from the dominant ICP pulse closest in time to the timing of LPR measurement. Then the slope of the fitted line would be accepted as a rate of change for the corresponding ICP metric, if its p (indicator of goodness of the fit as described in the previous subsection) is smaller or equal to thres p. Hence, based on the above probability threshold test, a trend of change is assigned to each ICP metric during an LPR episode as either positive “+” (a statistically significant positive slope) or negative “−”(a statistically significant negative slope) or zero “0” (a statistically non-significant slope).
2.4. Verification of the Consistency of the Trend of Change during the Identified Episodes of LPR Increase
Employing the trend analysis described before, each individual ICP metric may result in different trends (positive, negative or zero) during different LPR increase episodes. Therefore, a method needs to be developed to quantify the consistency of such a trend in association with LPR increase for a patient. In the current work, we use a binomial test to examine whether a MOCAIP ICP metric has a consistent trend (increasing or decreasing) with a probability greater than odds of 1/3 with 95% confidence. For this purpose, the percentage of the LPR episodes resulted in a positive or negative trend is calculated for each patient and each ICP metric. Then these percentages are compared with a threshold ξ obtained from the inverse of the binomial cumulative distribution function with success rate of 1/3 and number of trials equal to the number of LPR increase episodes. A trend is considered as a consistent one, if the percentage of LPR increase episodes where the corresponding ICP metric has a positive (or negative) slope is greater than ξ, indicating that observing such a trend for a MOCAIP metric during episodes of LPR increase is beyond a random occurrence.
2.5. Patient Data
The study group consists of 19 severe TBI patients (2 females, 17 males) with average age of 41.9 ± 22.7 years old. The patients had both ICP and microdialysis measurement during their stay at UCLA medical center and consented for allowing their data to be analyzed under the protocol as approved by the UCLA Internal Review Board.
The patients received continuous intracranial pressure monitoring for the clinical purpose using ventricular catheter. Simultaneous cardiovascular monitoring was performed using the bedside GE monitors. ICP and lead II of ECG signals were archived using the BedMaster system that collects data (sampling frequency of 240 Hz) from the GE Unity network to which the bedside monitors were connected to.
The Cerebral microdialysis was performed using the CMA70 probe (10 cm flexible shaft, 10mm membrane length, 20 kDa cutoff, CMA, Stockholm, Sweden) inserted via a twist drill burr hole adjacent to an existing ventriculostomy. The microdialysis catheter was inserted into a depth of 1.5 to 2cm below the dura at an angle 30 degrees lateral to the trajectory of the ventriculostomy, to place the catheter tip into the white matter in the nondominant frontal lobe. The probe was tunneled 3cm under the skin and secured to the scalp with a flat profile, and then attached to the CMA103 perfusion pump. Normal saline was perfused through the catheter at 2 mL/min, and fluid was collected (to control for systemic changes in glucose, lactate and pyruvate) and then placed into dry ice or directly into the CMA600 instrument. Then the lactate/pyruvat ratio were calculated for each existing MD measurement.
2.6. Data Analysis and Validation Protocol
MOCAIP algorithm was applied to an hour of ICP data around each MD measurement. As a result, a representative ICP pulse (called dominant pulse) was calculated from every 4 minutes of consecutive ICP data using a clustering method. For each dominant ICP pulse, six landmarks were identified and 128 pulse morphological metrics were extracted. All LPR increase episodes were identified using the method described in subsection 2.2 and a trend of change was assigned to each ICP metric during the corresponding episode. Then the binomial test of subsection 2.5 was applied to verify whether each ICP metric has a consistent trend accompanying LPR increase episodes.
3-Results
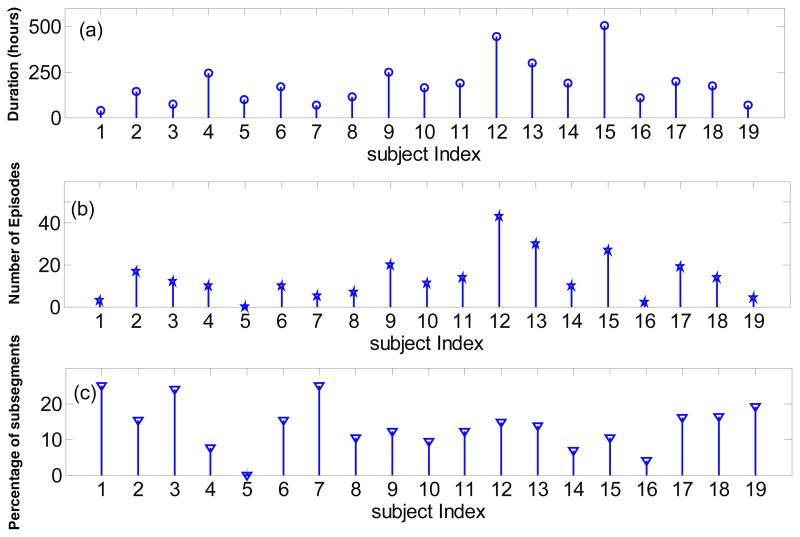
2261 microdialysis data samples were collected in an average interval of 1.7 ± 0.4 hours from 19 patients. The mean and standard deviation of the number of collected MD samples per patient is (119 ± 80 ). As figure1-A shows, there is a considerable inter-subject variability on the time duration of MD measurements (mean of 185 ± 122 hours). The mean of the measured LPR over all subjects of the study is 31.45 ± 16.81.
Figure 1.
Microdialysis data and episodes of Lactate/Pyruvate ratio (LPR) increase for all the subjects. (a) Time duration of microdialysis measurements in hours; (b) Number of the automatically identified LPR increase episodes; (c) Percentage of subsegments with length of 10 samples which are identified as an episode of LPR increase using the proposed method.
Under the baseline parameters, we found a total of 258 episodes of LPR increase. We note that the highest percentage of sub-segments identified as an LPR increase episode using the proposed method are obtained from patients #1 and 7, while no such episode has been identified for patient #5.
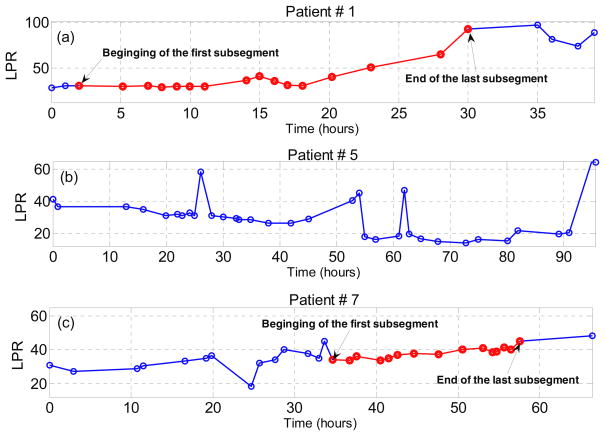
The LPR measurements for patients # 1, 5 and 7 have been demonstrated in Fig. 2 and the beginning and the end of the first and last LPR increase episodes have been identified (the sample points which belong to at least one of the identified LPR increase episodes are highlighted with the red color). We observe that LPR data of patient #5 does not include any increase episode of 10 samples with time range of 10 to 20 hours, while the LPR data of the other two subjects contains several of such episodes.
Figure 2.
The microdialysis data (LPR) measured over the time of study for three patients. All the sample points which belong to at least one automatically identified LPR increase episodes are highlighted with the red color. (a) patient #1; (b) patient # 5; and (c) patient #7.
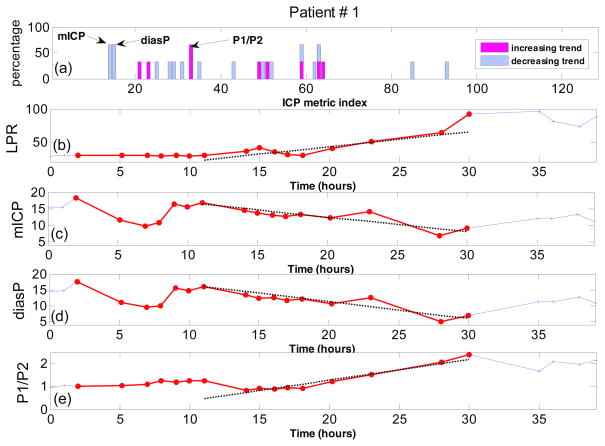
The results of the trend analysis of LPR time series for subject #1 are presented in Fig. 3. Plot A shows the percentage of LPR increase episodes where the corresponding ICP metric has either a positive (increasing), negative (decreasing) or zero trend (no-trend). We observe that few ICP metrics for subject #1 have a high percentage of episodes with an increasing/decreasing trend. In fact, implementing the consistency analysis with ξ = 66% reveals that the 14th and 15th ICP metrics (mean ICP and diastolic pressure) have a consistent decreasing trend, while the 33rd ICP metric (the ratio of the first peak to the second peak of the ICP pulse) has a consistent increasing trend. Plots C through E show these three metrics over the time, during the identified LPR increase episodes (Plot B). As these figures show, mean ICP and diastolic pressure start to p decrease from around 20 mmHg to below 10 mmHg, while the ratio of increases from 1 to 2.4. The dotted black line on plots B through E is the line fitted to the corresponding data during the last LPR increase episode, using the weighted least square method described in subsections 2.2 and 2.3.
Figure 3.
Trend analysis for patient #1. (a) Percentage of LPR increase episodes with increasing or decreasing trend for all 128 ICP metrics; (b) The measured LPR over the time; (c) mean ICP (mmHg) over time; (d) diastolic pressure (mmHg) over time; (e) the ratio of the first peak to the second peak of the ICP pulse over time. All the sample points which belong to at least one automatically identified LPR increase episodes are highlighted with the red color. The dotted lines on (b), (c), (d) and (e) are the regression lines robustly fitted to the corresponding data during the last identified episode of LPR increase (length of 10 samples).
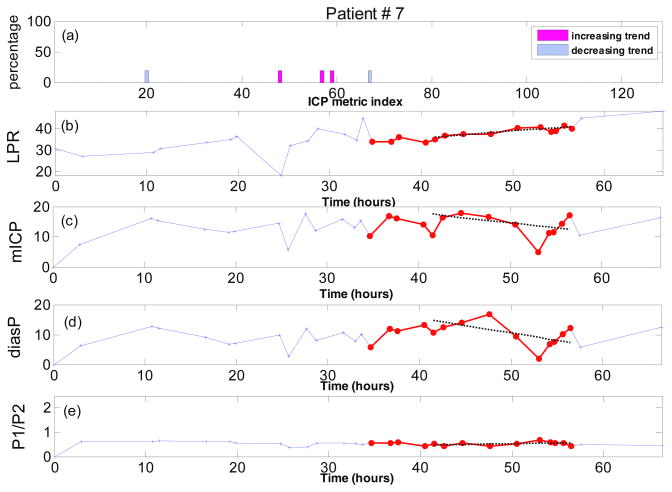
Performing the same trend analysis for patient #7 (Fig. 4) reveals that most of the ICP metrics show no trend and even for the few metrics which demonstrate an increasing or decreasing trend, the percentage of episodes with those trends are very small (figure 4(A)). This is such that none of the ICP metrics of subject #7 passes the binomial test for the consistency of the metric trend. The no-trend conclusion for the ICP metrics of this subject can be confirmed from figures 4(C) through 4(E) where the values of the three metrics (mean ICP and diastolic pressure and ) have been shown over the time. We observe that the discrepancies between the values of these MOCAIP metrics and those expected from the fitted lines are large due to the lack of a monotonic trend. As a result, a “zero trend” is assigned to these three metrics.
Figure 4.
Trend analysis for patient #7. (a) Percentage of LPR increase episodes with increasing or decreasing trend for all 128 ICP metrics; (b) The measured LPR over the time; (c) mean ICP (mmHg) over time; (d) diastolic pressure (mmHg) over time; (e) the ratio of the first peak to the second peak of the ICP pulse over time. All the sample points which belong to at least one automatically identified LPR increase episodes are highlighted with the red color. The dotted lines on (b), (c), (d) and (e) are the regression lines robustly fitted to the corresponding data during the last identified episode of LPR increase (length of 10 samples).
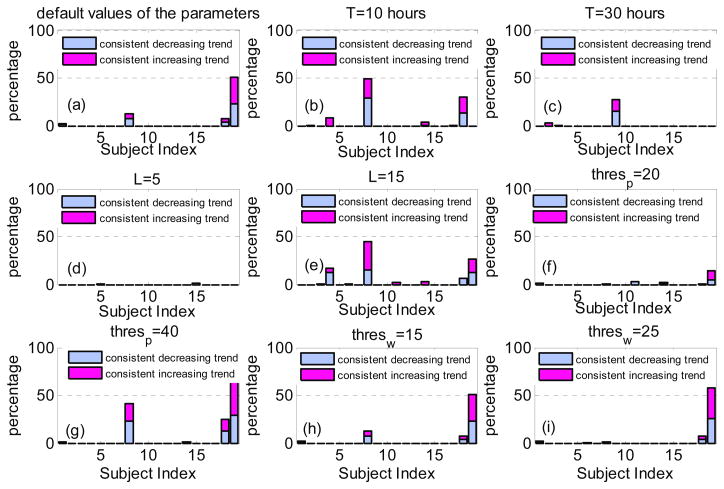
The percentages of the ICP metrics with consistent trend for all the subjects in the study using default values of the parameters ( L = 10, T 20 hours, thres p = 30th percentile and thres w = 5th percentile ) have been presented in figure 5(A). We observe that for 15 subjects, there is no ICP metric with a consistent trend over all episodes of LPR increase. For subjects #1, #8, and #18, there exist only few ICP metrics (less than 17 metrics) with consistent trend, while half of the ICP metrics for subject #19 demonstrate either a consistent increasing or consistent decreasing trend.
Figure 5.
The percentage of the ICP metrics with consistent increasing (or consistent decreasing trend) for all the subjects in the study using different values of the parameters. (a)The default values of the parameters as (L = 10, T = 20, thres p = 30th percentile and thres w = 5th percentile); (b) decreasing T, ( L = 10, T = 10, thres p = 30th percentile and thres w = 5th percentile); (c) increasing T, ( L = 10, thres p, T = 30, thres p = 20th percentile and thres w = 5th percentile); (d) decreasing L, ( L = 5, T = 10, thres p = 40th percentile and thres p = 5th percentile); (e) increasing L, (L = 15, T = 10, thres p 40th percentile and thres w = 5th percentile); (f) decreasing thres p, ( L = 10, T = 10, thres p = 20th percentile and thres w = 25th percentile); (g) increasing thres p, (L = 10, T = 10, thres p = 40th percentile and thres w = 25th percentile); (h) increasing, thres w, (L = 10, T = 10, thres p = 30th percentile and thres w = 15th percentile); (i) increasing thres w, ( L = 10, T = 10, thres p = 30th percentile and thres w = 25th percentile).
To investigate the effect of the values of different data analysis parameters on the results of the presented study, we repeated the same analysis for T = 10 hours (Fig. 5(B)), T = 30 hours (Fig. 5(C)), L = 5 samples (Fig. 5(D)), L = 15 samples (Fig. 5(E)), thres p = 20th percentile (Fig. 5(F)), thres p = 40th percentile (Fig 5. (G)), thres w = 15th percentile (Fig. 5(H)) and thres w = 25th percentile (Fig. 5(I)). We note that choosing different values for different parameters may change the percentage of those ICP metrics with consistent trend for some of the patients. But for the majority of the subjects of the study (14 subjects), most of the ICP metrics (more than 95%) do not show any consistent trend during the episodes, regardless of the chosen values for the parameters.
More investigation reveals that the only ICP metric with a consistent trend over all the aforementioned cases of subject #8 is mICP (decreasing trend), while there is no common ICP metrics with a consistent trend over all the aforementioned cases of subject #18. The ICP metrics with a consistent trend over the cases of subject #19 is pulse latency (increasing trend), diastolic pressure (decreasing trend) and the ratio of the second peak to the third peak of the ICP pulse (decreasing trend).
As a result, even for the five patients whose percentage of ICP metrics with consistent trend was above 5% for some parameters values, there exists no common ICP metric which is consistently increasing or decreasing over the LPR increase episodes.
4-Discussion
For the last two decades, cerebral microdialysis has become a commercially available clinical tool to monitor brain biochemical process during neurointensive care (Meyerson et al., 1990). MDis primarily focused on markers of cerebral energy metabolism (glucose, lactate, and pyruvate), cell damage (glycerol), and neurotransmitters (glutamate) (Ungerstedt and Rostami, 2004). Some studies have shown that the lactate/pyruvate ratio could be a sensitive indicator of changes in the redox state of cells caused by cerebral hypoxia or ischemia (Tisdall and Smith, 2006; Kawai et al.; Hillered et al., 2005). Other studies have demonstrated that during hypoglycemic events, cerebral blood flow increases and this implies that the increased LPR is unlikely to be ischemia-related (Choi et al., 2001; Kennan et al., 2005). Consequently, the context of an increase in LPR should be considered carefully before any conclusions about its pathophysiology are drawn (Larach et al.). In fact, establishing such a context to explain LPR increase would provide a viable clinical decision support for prescribing appropriate actions to avert further LPR increase beyond dangerous level. This vision motivated the investigation of ICP pulse morphological changes in association with LPR increase for the present work.
ICP is an established monitoring modality for neurocritical care and realizing its potential to provide additional real-time information concerning cerebrovascular changes is becoming feasible based on a recently developed ICP pulse morphological analysis algorithm (Hu et al., 2009) and its two applications of; 1) using derived ICP pulse morphological features to detect cerebral hypoperfusion (as Xenon133 CBF < 20) (Hu et al.) and; 2) to assess acute cerebral vasodilatation caused by cerebral hypercapnia (Asgari et al., 2011), respectively. Since a consistent ICP pulse morphological changes is a necessary condition for a mono-phasic cerebral vascular change such as cerebral vasoconstriction or vasodilatation, the lack of such consistent ICP pulse morphological changes during LPR increase would help rule out a cerebral vascular cause of LPR increase.
Our results suggest mono-phasic cerebral vascular changes are probably not a frequent reason for observed LPR increase in the TBI patients studied. This conclusion is in agreement with those of some other existing studies(Vespa et al., 2007; Nelson et al., 2004; Bjerring et al.), which were not able to find an association between LPR increase and ICP, cerebral perfusion pressure, and cerebral ischemia. So we believe that factors other than cerebrovascular nature and cerebral hemodynamics seem to be primarily responsible for LPR increase in TBI patients (Larach et al., 2011). This conclusion may seem to be in contrast with those of the recent work of (Timofeev et al., 2011a). Please note that although the presented work and that of (Timofeev et al., 2011a) are related, their goals and approaches are completely different. The present work tries to investigate whether there exist any consistent morphological changes (rather than just focusing on the mean value) of the ICP signal when LPR increases over time, while the authors in (Timofeev et al., 2011a) try to find the correlation of mean ICP, LPR and other related parameters with the outcome of the patients. So in some sense, the present work looks at the dynamic changes of ICP over time and its correlation with LPR within individual patient, where the focus of the (Timofeev et al., 2011a) has been on the association between overall values of ICP and LPR and the outcome across patients.
We acknowledge that changing several data analysis parameters (as illustrated in Fig.5) for automatic identification of an LPR increase episode may affect the number of identified episodes and consequently the percentages of the time when a specific MOCAIP metric is correlated with the LPR increase or the number of such metrics with a consistent trend. But for majority of the subjects in the study, there is still no significant correlation between any of the ICP metrics and the LPR increase and hence the qualitative results obtained under the baseline parameters still remain valid.
It should be also noted that MD values were obtained on an hourly basis. As such, one limitation of the present study would be related to the mismatched temporal resolutions of MD and ICP pulse morphological features. Cerebrovascular changes (vasoconstriction) can occur within a short period of time and stay unchanged. As a consequence, LPR can still gradually increase after completion of cerebrovascular changes, which would result in inconsistent ICP pulse morphological changes over the long period. The current experiment design will not be able to rule out these short-term cerebrovascular alternations as possible reason for LPR increase.
We should be also cautious in our usage of a recent finding that acute cerebral vasodilatation caused by hypercapnia is associated with consistent ICP pulse morphological changes (Asgari et al., 2011) to establish the consistent ICP pulse morphological changes as a necessary condition for any mono-phasic cerebrovascular alternations. Acute cerebral vasodilatation developed within minutes as hypercapnia was introduced through inhaling from a CO2 gas mixture. In the present work, we were investigating ICP pulse morphological changes in a period of hours. This time-scale mismatch may have invalidated establishing cerebrovascular changes based on the criterion of consistent ICP pulse morphological changes. LPR is a local measure of brain biochemical process while ICP pulses morphology reflects global changes. This factor may be also responsible for the lack of observing consistent ICP pulse morphological changes in association with LPR increase.
5-Conclusion
For the majority of the subjects of the study, none of the ICP metrics show any consistent trend during the LPR increase episodes of at least 5 hours. Even for the few remaining subjects, the number of ICP metrics with a consistent trend is very small and those metrics varies from subject to subject. In conclusion, no clear relationship was found between the LPR increase and the alternation of the intracranial pressure pulse morphology.
Acknowledgments
The present work is partially supported by NINDS awards (NS059797, NS054881 and NS066008) and UC Brain Injury Research Center (BIRC).
References
- Adolph RJ, Fukusumi H, Fowler NO. Origin of cerebrospinal fluid pulsations. The American journal of physiology. 1967;212:840–6. doi: 10.1152/ajplegacy.1967.212.4.840. [DOI] [PubMed] [Google Scholar]
- Asgari S, Bergsneider M, Hamilton R, Vespa P, Hu X. Consistent changes in intracranial pressure waveform morphology induced by acute hypercapnic cerebral vasodilatation. Neurocritical care. 2011;15:55–62. doi: 10.1007/s12028-010-9463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S, Bergsneider M, Hu X. A robust approach toward recognizing valid arterial-blood-pressure pulses. IEEE Trans Inf Technol Biomed. 2010;14:166–72. doi: 10.1109/TITB.2009.2034845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S, Xu P, Bergsneider M, Hu X. A subspace decomposition approach toward recognizing valid pulsatile signals. Physiological measurement. 2009;30:1211–25. doi: 10.1088/0967-3334/30/11/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerring PN, Hauerberg J, Jorgensen L, Frederiksen HJ, Tofteng F, Hansen BA, Larsen FS. Brain hypoxanthine concentration correlates to lactate/pyruvate ratio but not intracranial pressure in patients with acute liver failure. Journal of hepatology. 2010;53:1054–8. doi: 10.1016/j.jhep.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Cardoso ER, Reddy K, Bose D. Effect of Subarachnoid Hemorrhage on Intracranial Pulse Waves in Cats. Journal of Neurosurgery. 1988;69:712–8. doi: 10.3171/jns.1988.69.5.0712. [DOI] [PubMed] [Google Scholar]
- Cardoso ER, Rowan JO, Galbraith S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg. 1983;59:817–21. doi: 10.3171/jns.1983.59.5.0817. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab. 2001;21:653–63. doi: 10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Guazzo E, Whitehouse M, Smielewski P, Czosnyka Z, Kirkpatrick P, Piechnik S, Pickard JD. Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir (Wien) 1996;138:531–41. doi: 10.1007/BF01411173. discussion 41–2. [DOI] [PubMed] [Google Scholar]
- Eide PK. A new method for processing of continuous intracranial pressure signals. Med Eng Phys. 2006;28:579–87. doi: 10.1016/j.medengphy.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ellis T, McNames J, Aboy M. Pulse morphology visualization and analysis with applications in cardiovascular pressure signals. Ieee Transactions on Biomedical Engineering. 2007;54:1552–9. doi: 10.1109/TBME.2007.892918. [DOI] [PubMed] [Google Scholar]
- Hadi AS, Simonoff JS. Procedures for the Identification of Multiple Outliers in Linear Models. Journal of the American Statistical Association. 1993;88:1264–72. [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. Journal of neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Goodman JC, Contant CF, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. Journal of neurotrauma. 2004;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- Holland PW, Welsch RE. Robust Regression Using Iteratively Reweighted Least-Squares. Communications in Statistics: Theory and Methods. 1977;A6:813–27. [Google Scholar]
- Hu X, Glenn T, Scalzo F, Bergsneider M, Sarkiss C, Martin N, Vespa P. Intracranial pressure pulse morphological features improved detection of decreased cerebral blood flow. Physiological measurement. 31:679–95. doi: 10.1088/0967-3334/31/5/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xu P, Asgari S, Paul V, Bergsneider M. Forecasting ICP Elevation Based on Prescient Changes of Intracranial Pressure Waveform Morphology. IEEE Transactions on Biomedical Engineering. 2010 doi: 10.1109/TBME.2009.2037607. Accepted. [DOI] [PMC free article] [PubMed]
- Hu X, Xu P, Scalzo F, Vespa P, Bergsneider M. Morphological clustering and analysis of continuous intracranial pressure. IEEE transactions on bio-medical engineering. 2009;56:696–705. doi: 10.1109/TBME.2008.2008636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz M, Asgari S, Bergsneider M, Czosnyka M, Hamilton R, Hu X. Pattern recognition of overnight intracranial pressure slow waves using morphological features of intracranial pressure pulse. J Neurosci Methods. 190:310–8. doi: 10.1016/j.jneumeth.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Kawakita K, Yano T, Abe Y, Kuroda Y, Tamiya T. Use of intracerebral microdialysis in severe traumatic brain injury. No shinkei geka. 38:795–809. [PubMed] [Google Scholar]
- Kennan RP, Takahashi K, Pan C, Shamoon H, Pan JW. Human cerebral blood flow and metabolism in acute insulin-induced hypoglycemia. J Cereb Blood Flow Metab. 2005;25:527–34. doi: 10.1038/sj.jcbfm.9600045. [DOI] [PubMed] [Google Scholar]
- Laitinen L. Origin of arterial pulsation of cerebrospinal fluid. Acta neurologica Scandinavica. 1968;44:168–76. doi: 10.1111/j.1600-0404.1968.tb05563.x. [DOI] [PubMed] [Google Scholar]
- Larach DB, Kofke WA, Le Roux P. Potential Non-Hypoxic/Ischemic Causes of Increased Cerebral Interstitial Fluid Lactate/Pyruvate Ratio: A Review of Available Literature. Neurocritical care. 2011 doi: 10.1007/s12028-011-9517-8O. (Epub ahead of print) [DOI] [PubMed]
- Meyerson BA, Linderoth B, Karlsson H, Ungerstedt U. Microdialysis in the human brain: extracellular measurements in the thalamus of parkinsonian patients. Life sciences. 1990;46:301–8. doi: 10.1016/0024-3205(90)90037-r. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Bellander BM, Maccallum RM, Axelsson J, Alm M, Wallin M, Weitzberg E, Rudehill A. Cerebral microdialysis of patients with severe traumatic brain injury exhibits highly individualistic patterns as visualized by cluster analysis with self-organizing maps. Critical care medicine. 2004;32:2428–36. doi: 10.1097/01.ccm.0000147688.08813.9c. [DOI] [PubMed] [Google Scholar]
- Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- Scalzo F, Asgari S, Kim S, Bergsneider M, Hu X. Robust peak recognition in intracranial pressure signals. Biomed Eng Online. 2010;9:61. doi: 10.1186/1475-925X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo F, Xu P, Asgari S, Bergsneider M, Hu X. Regression analysis for peak designation in pulsatile pressure signals. Medical & Biological Engineering & Computing. 2009;47:967–77. doi: 10.1007/s11517-009-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim O, Vik A, Gulati S, Eide PK. Rapid and severe rise in static and pulsatile intracranial pressures during a generalized epileptic seizure. Seizure. 2008;17:740–3. doi: 10.1016/j.seizure.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Carpenter KL, Nortje J, Al-Rawi PG, O’Connell MT, Czosnyka M, Smielewski P, Pickard JD, Menon DK, Kirkpatrick PJ, Gupta AK, Hutchinson PJ. Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain. 2011a;134:484–94. doi: 10.1093/brain/awq353. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Czosnyka M, Carpenter KL, Nortje J, Kirkpatrick PJ, Al-Rawi PG, Menon DK, Pickard JD, Gupta AK, Hutchinson PJ. Interaction between Brain Chemistry and Physiology after Traumatic Brain Injury: Impact of Autoregulation and Microdialysis Catheter Location. Journal of neurotrauma. 2011b;28:849–60. doi: 10.1089/neu.2010.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MM, Smith M. Cerebral microdialysis: research technique or clinical tool. British journal of anaesthesia. 2006;97:18–25. doi: 10.1093/bja/ael109. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Rostami E. Microdialysis in neurointensive care. Current pharmaceutical design. 2004;10:2145–52. doi: 10.2174/1381612043384105. [DOI] [PubMed] [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–74. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, O’Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, Glenn T, Hovda DA. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Critical care medicine. 2007;35:1153–60. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]