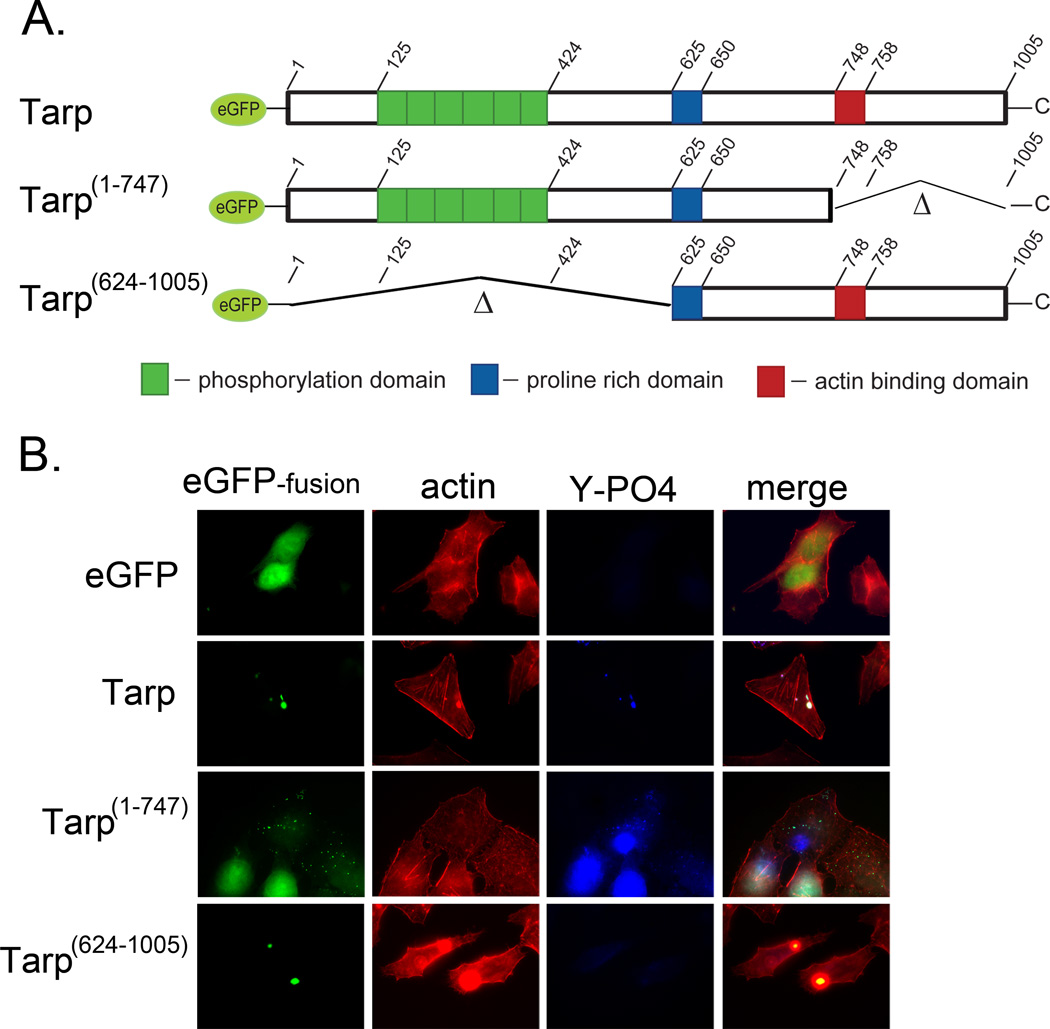
Fig. 2.
Actin filaments co-localize with ectopically expressed Tarp in a phosphorylation domain independent manner. Tarp proteins ectopically expressed as enhanced green fluorescence protein fusions were examined for their ability to localize with actin filaments. (A) Schematic of the eGFP-Tarp fusion proteins indicating the location of the actin binding domain (red box), the proline rich domain (blue box) and the tyrosine rich phosphorylation domain (green boxes). Δ indicates amino acids deleted in the mutant eGFP –Tarp fusion proteins and numbers indicate amino acid positions encoded within the C. trachomatis tarP gene (B) Host cells expressing eGFP (eGFP) alone or eGFP fusions (eGFP-fusion) of full length Tarp (Tarp), or deletion mutants lacking the phosphorylation domain (Tarp 624–1005) or actin binding domain (Tarp 1–747) but harboring the proline rich domain (PRD) required for protein aggregation were fixed and stained with Alexa fluor 568 conjugated phalloidin (actin), phosphotyrosine specific antisera (Y-PO4) and goat anti-mouse conjugated to Alexa 350.