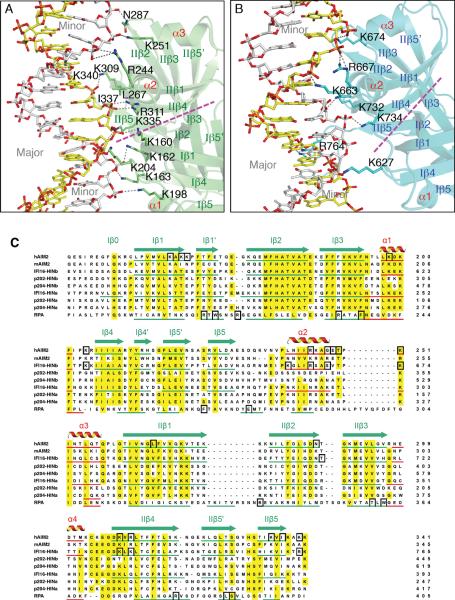
Figure 3. The HIN Domains Bind Both Strands of The dsDNA.
(A) Detailed HIN:DNA interactions for the AIM2 HIN:DNA complex. The hydrogen bonds are indicated as gray dotted lines. Secondary structures for the AIM2 HIN domain (lime) are labeled and the two DNA strands are colored yellow and silver, respectively. The approximate boundaries of the OB1-OB2 are marked with a magenta dotted line and the major and minor grooves of the dsDNA are marked in gray.
(B) Detailed HIN:DNA interactions are shown for the IFI16 HINb:DNA complex similar to (A), except the IFI16 HINb domain is colored cyan.
(C) Sequence alignment of the HIN domains. Sequences of selected dsDNA-binding HIN domains from human AIM2 (NP_004824), mouse AIM2 (NP_001013801), human IFI16 (Q16666), mouse p204 (NP_032355, a homolog of human IFI16), mouse p202 (NP_032353, an inhibitor of AIM2), as well as a ssDNA-binding OB superfamily protein RPA (NP_002936) were aligned by ClustalW (Thompson et al., 1994) with minor adjustments. The α helices are in red, and the β strands were underlined in green and marked with “I” and “II” for OB1 and OB2, respectively. Conserved residues are shaded in yellow, and DNA binding residues in black boxes.
See also Figure S2.