Fig. 2.
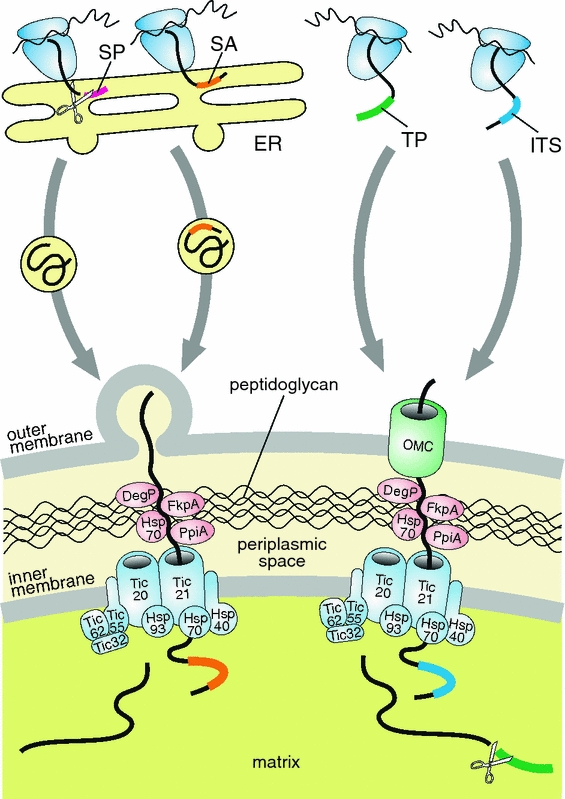
Hypothetical import pathways of nuclear-encoded proteins into the cyanobacterial endosymbionts of Paulinella chromatophora. One model postulates that these proteins carry an N-terminal signal peptide (SP) or signal anchor (SA) and are targeted to the endosymbionts in vesicles derived from the endoplasmic reticulum (ER) (left panel). However, by analogy with classical primary plastids and mitochondria, we cannot exclude the possibility that some proteins are equipped with a transit peptide (TP) or an internal targeting signal (ITS), which results in their translocation via some outer membrane channel (OMC) such as Omp85/Toc75, Tom40, or Tim22/OEP16 (right panel). Proteins released into the intermembrane space could migrate through the peptidoglycan wall freely, or with the assistance of molecular chaperones homologous to the higher plant Hsp70 (DnaK) as well as the bacterial DegP, FkpA, and PpiA, which are encoded in endosymbiont’s genome (Bodył et al. 2010). Protein translocation across the inner endosymbiont membrane could be mediated by a Tic-like translocon characteristic of classical primary plastids because the Paulinella endosymbiont genome encodes significant homologs to several Tic proteins (Bodył et al. 2010). The homologs of Hsp93, Hsp70, and Hsp40 could provide a pulling force to import proteins into the endosymbiont matrix
