Table 2.
Synthesis of cyclohexenone derivatives via Rh-catalyzed 1,3-acyloxy migration, [4 + 2] cycloaddition, and hydrolysisa
| Entry | Substrate | Product | Yield |
|---|---|---|---|
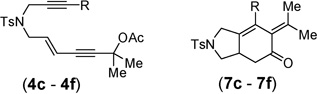 |
|||
| 1 | R = H, 4c | 7c | 95% |
| 2 | R = Me, 4d | 7d | 95% |
| 3 | R = Ph, 4e | 7e | 92% |
| 4 | R = 4-BrC6H4, 4f | 7f | 87% |
| 5 | 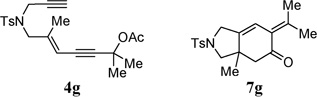 |
90% | |
| 6 | 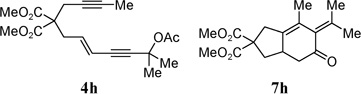 |
86% | |
| 7 | 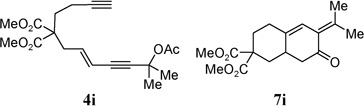 |
95% | |
| 8 | 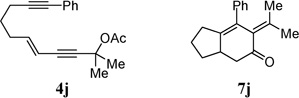 |
91% | |
| 9 | 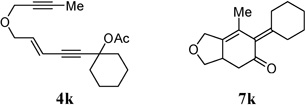 |
86% | |
 |
|||
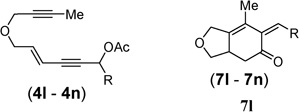
| 10b,d | R = t-Bu, 4l | 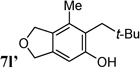 |
(Z/E > 20 : 1)cZ-7l 61% 7l′: 30% |
| 11b | R = i-Pr, 4m | 7m | (Z/E = 3.7 : 1)cZ-7m 58% |
| 12b | R = CH2OTBS, 4n | 7n | (Z/E = 3 : 1)cZ-7n 52% |
Condition A: (1) [Rh(CO)2Cl]2 (5 mol%), DCE, 80 °C, 5–20 min; (2) K2CO3, MeOH.
Condition B: (1) [Rh(CO)2Cl]2 (5 mol%), [(CF3)2CHO]3P (20 mol%), DCE, 80 °C, 15–20 min; (2) K2CO3, MeOH.
The ratio was determined by 1H NMR of the crude product.
The substrate was heated at 80 °C for 45 min under condition B.
