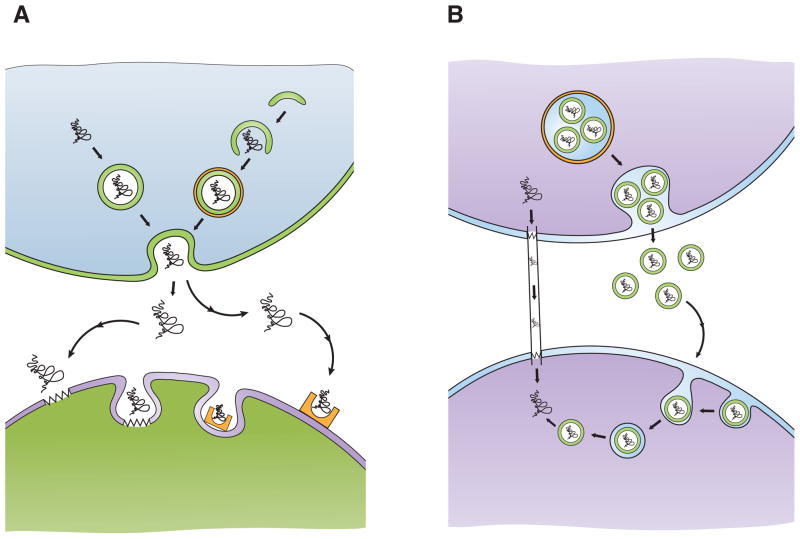
Figure 4. Proposed mechanisms to account for cell-to-cell transmission of misfolded proteins.
A) Exocytosis/endocytosis. Misfolded proteins can be packaged into exocytotic vesicles (top left; arrows), which fuse with the cell membrane and release their contents into the extracellular space. Misfolded proteins may be delivered to pre-autophagic vesicles that mature into double-membrane bound autophagosomes (top right; arrows). One possible fate of certain autophagosomes is to fuse with the cell membrane and release their contents into the extracellular space. Once in the extracellular space, misfolded proteins can be taken up by cells via lipid raft-mediated endocytosis (bottom left; zigzags represent lipid rafts), or more likely by receptor-mediated endocytosis (bottom right; half rectangle with oval indentation represents receptor).
B) Propagation without membrane-bound transport. Misfolded proteins may move from one cell to another via intercellular bridges, known as “tunneling nanotubes” (left; arrows). Another route for cell-to-cell transfer of misfolded proteins is within exosomes, small membrane-bound vesicles (green circles) that can form within multivesicular bodies (orange circle), which are released and endocytosed by a neighboring cell (right; arrows). Such exosomes can bud off of their original cell and travel large distances before fusing with the membrane of another cell. How misfolded protein conformers, sequestered in endosomes, gain access to the cytosolic compartment remains unclear.