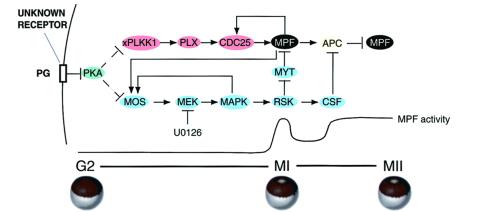
Figure 1.
Signal transduction pathways regulated by progesterone in meiosis. The model depicts progesterone acting through an unknown membrane receptor to inhibit adenylyl cyclase, reduce the level of cAMP, and decrease the activity of PKA. Through multiple unknown steps decreased PKA leads to translational activation of Mos mRNA and subsequent generation of an active MAPK pathway. A target of MAPK, p90RSK, operates in meiosis I (MI) to promote MPF activation by inhibition of Myt1, and in meiosis II (MII) p90RSK inhibits cyclin B degradation by the anaphase-promoting complex (APC) as an element required for cytostatic factor (CSF)-mediated metaphase arrest. Decreased PKA also leads to activation of the polo-like kinase cascade by multiple unknown steps. This kinase cascade pathway results in activation of Cdc25C, the phosphatase that dephosphorylates and activates MPF (cyclin B/Cdc2) at the G2/M transition in meiosis I. Feedback loops exist in which activated MPF can directly activate Cdc25, and Mos mRNA translation and/or stability can be increased by active MPF and active MAPK via multiple unknown steps. PG, progesterone; xPlkk1, Xenopus polo-like kinase kinase; Plx, Xenopus polo-like kinase; MEK, MAP kinase kinase.