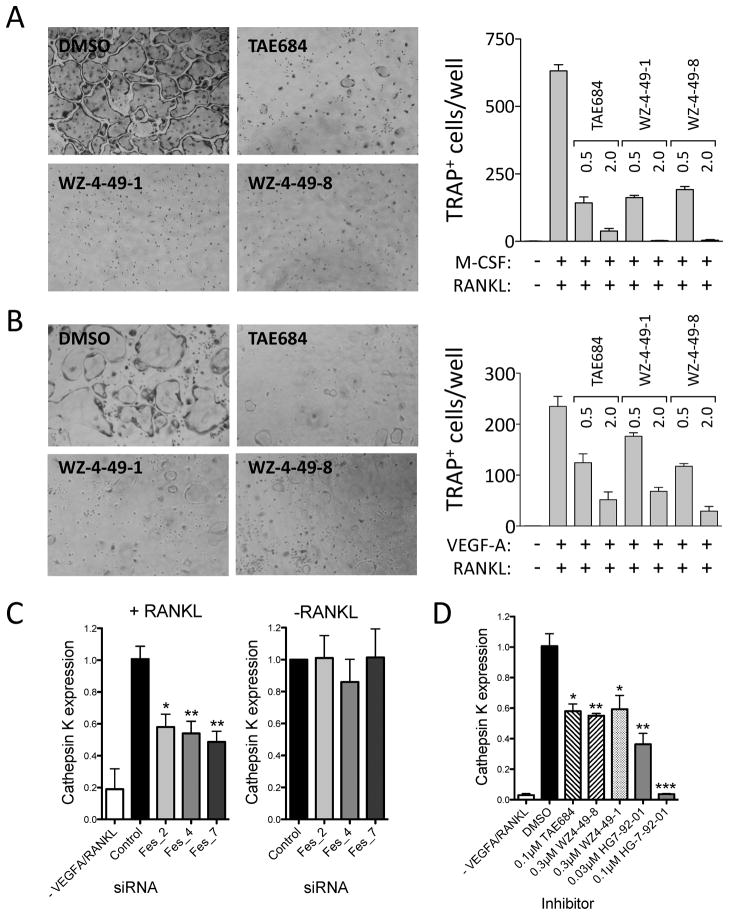
Figure 6. c-Fes inhibitors suppress osteoclast differentiation from bone marrow-derived macrophages (BMM) and RAW 264.7 cells and reduce RANKL-induced upregulation of the osteoclast marker Cathepsin K.
Mouse BMM (A) and RAW 264.7 mouse macrophages (B) were cultured with 100 ng/ml RANKL and M-CSF (BMM) or VEGF-A (RAW 264.7 cells) to induce osteoclast formation in the presence of the indicated c-Fes inhibitors at 0.5 and 2.0 μM. Control cultures without inhibitors were supplemented with the DMSO carrier solvent (0.1%). Cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) activity. Representative fields of DMSO and inhibitor-treated cells are shown on the left. TRAP-positive multinuclear cells were counted from three independent cultures, and the mean values are plotted on the right ± SD. Three independent experiments produced comparable results. All inhibitor-treated cultures exhibited significantly fewer differentiated cells relative to the DMSO-treated control (p < 0.05 in each case). In additional experiments, RAW264.7 cells transfected with c-Fes specific siRNAs were grown in the presence of VEGF-A and RANKL for 6 days (C, left panel) or in the absence of cytokines for 24 h (C, right panel). RNA was isolated and levels of the osteoclast marker Cathepsin K were analyzed by real-time quantitative RT-PCR. Mean expression values ± SEM of triplicate samples are plotted relative to control cells. Quantitative RT-PCR was used to measure Cathepsin K mRNA expression in cell populations stimulated for 6 days with RANKL in the presence of DMSO or the indicated concentrations of c-Fes inhibitors (Mean ± SEM, Figure 6D). See also Figure S6.