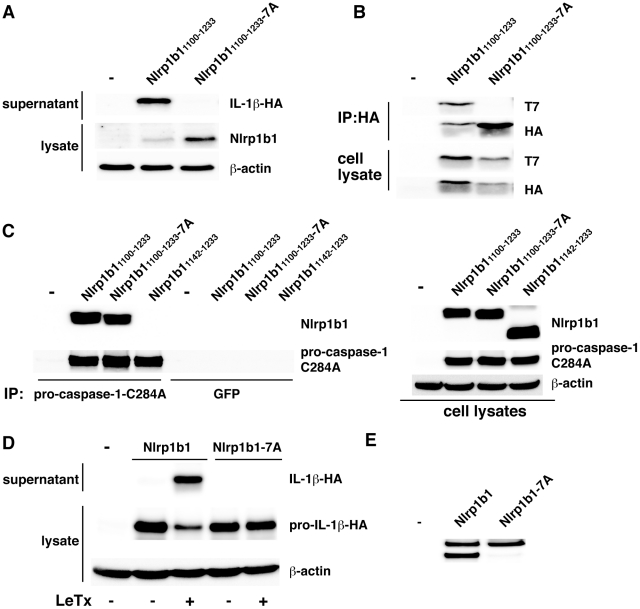
Figure 3. Mutation of amino acids 1100–1106 impairs FIIND self-association, FIIND cleavage, and Nlrp1b1 activity.
(A) Nlrp1b11100–1233 or Nlrp1b11100–1233-7A (containing alanine substitution mutations of amino acids 1100–1106) was expressed with pro-caspase-1 and pro-IL-1β-HA in HT1080 cells. Cells were lysed 24 h after transfection. Cell lysates were probed for T7-tagged Nlrp1b1 constructs and for β-actin by immunoblotting; supernatants were immunoprecipitated with anti-HA antibodies and then probed for HA-tagged IL-1β. (B) T7-tagged and HA-tagged Nlrp1b11100–1233 or Nlrp1b11100–1233-7A were expressed in HT1080 cells. HA-tagged proteins were immunoprecipitated from cell lysates and the immunprecipitates were probed for T7- and HA-tagged proteins. (C) HA-tagged Nlrp1b11100–1233, Nlrp1b11100–1233-7A and Nlrp1b11142–1233 were co-expressed with pro-caspase-1-C284A-T7. Immunoprecipitations were performed with anti-T7 or control anti-GFP antibodies and immunoblotted for HA and T7 epitopes. Cell lysates (right panel) were immunoblotted as indicated. (D) Nrlp1b1 and Nrlp1b1-7A were expressed with pro-caspase-1 and pro-IL-1β-HA in HT1080 cells. Cells were treated with LeTx for 3 h and supernatants were probed for IL-1β-HA as above. (E) Cell lysates from (D) were probed for Nlrp1b1. Blots are representative of three independent experiments.