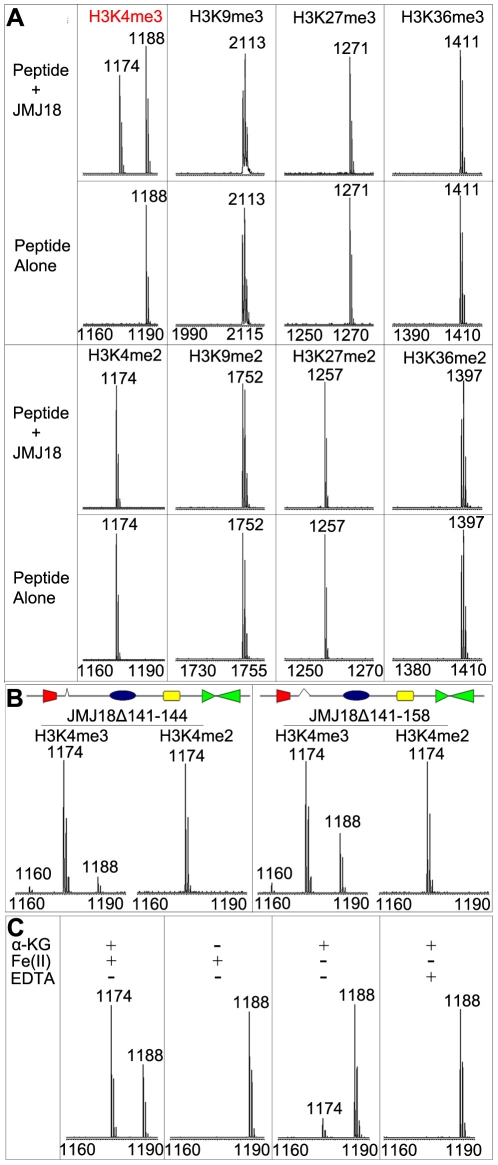
Figure 1. Mass spectrometric analysis of JMJ18 histone demethylase activity in vitro.
(A) Characterization of JMJ18 histone demethylase site specificity using purified full-length JMJ18. The numbers represent the molecular weights of the peptides. (B) Histone demethylase activity of two truncated forms of JMJ18: His-JMJ18Δ141–144 and His-JMJ18Δ141–158. Tri- and di-methylated H3K4 peptides were used in the assay. Both truncated JMJ18 were able to demethylate tri-methylated H3K4 to the di- and mono-methylated states; however, no demethylase activity was detected if di-methylated H3K4 peptide was used as the substrate. The deleted domain was indicated by an open arrow in the schematic structure of JMJ18. (C) The histone demethylase activity of JMJ18 depends on α-KG and Fe(II).