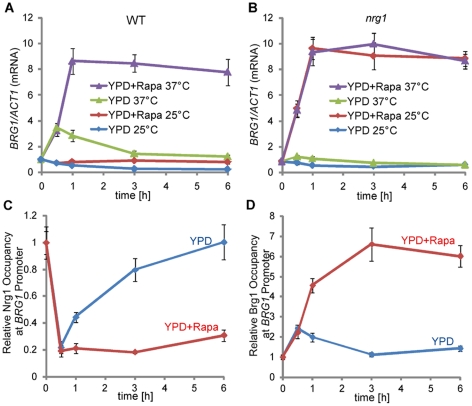
Figure 3. BRG1 expression is controlled by both removal of Nrg1 repression and rapamycin-dependent activation.
qRT-PCR analysis of BRG1 expression in wild type (A) and in the nrg1 mutant (B). Cells were diluted 1∶250 fold into pre-warmed YPD medium at 25°C or 37°C in the presence or absence of 10 nM rapamycin. BRG1 mRNA levels were determined by qRT-PCR. The signals obtained from ACT1 mRNA were used for normalization. The 0 h normalized value of BRG1/ACT1 for the wild type was set to be 1.00, and used for normalization of all other values in A and B. ChIP time courses of Nrg1-Myc (HLY3922) and Brg1-Myc (HLY4082) are shown in C and D, respectively. Cells were diluted into YEPD medium at 37°C in the presence or absence of 10 nM rapamycin. ChIP DNA were quantitated by qPCR with primers at −1959∼−1710 bp of the BRG1 promoter as described in Figure 1. All data show an average of three independent qRT-PCR or qPCR experiments with error bars representing the SEM.