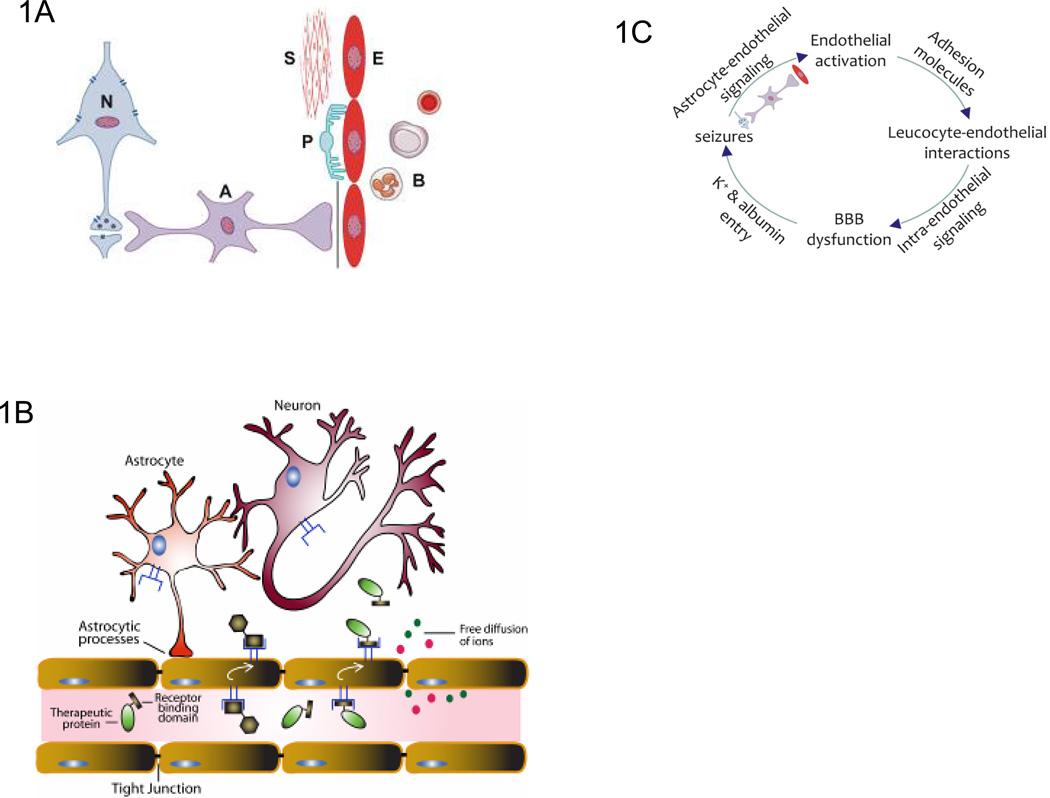
Figure 1. The extended Neurovascular Unit (NVU).
a. The blood-brain barrier (BBB) is an essential part of the neurovascular unit (NVU). A classical view of the NVU incorporates neurons, glial cells such as astrocytes and microglial cells closely juxtaposed with vascular endothelial cells, pericytes and smooth muscle cells. Blood cells, particularly PMN cells, lymphocytes and monocytes, also interact with the BBB endothelium and are therefore an integral part of this unit. The interactions between these cellular components and inter- and intra-cellular signaling regulate NVU function to maintain homeostasis, or to respond to inflammation and disease.
b. Receptor-mediated transcytosis of proteins at the BBB. Transcytosis is a receptor-mediated transport mechanism by which proteins that are targeted to the CNS bind extracellular receptors in vascular lumen, transport across the BBB endothelial cells, and are released in brain parenchyma. The presence of specific receptors (i.e. the insulin receptor) on the surface of BBB endothelial cells has allowed targeting and transport of some therapeutic proteins to the CNS44, 128.
c. Pathological signaling in the extended NVU. The proposed sequence order is based on data available from the epilepsy field144 and requires further exploration in the context of other brain diseases, including stroke and AD. The cycle starts with altered expression of vascular cell adhesion molecules and interactions of leucocytes with the endothelium, initiating intra-endothelial signals that alter BBB function and lead to neural tissue dysfunction as a consequence of K+ and albumin entry into the brain interstitium. Astrocytes detect the altered neuronal activity and transmit signals back to the BBB thereby facilitating interactions with leucocytes and turning the sequence into a vicious circle that maintains and exacerbates the pathological state. The activated endothelium may, as an integral part of the extended NVU, disturb neuron–astrocyte interactions, thereby adding an additional layer of pathological signaling to the process. Astrocytes emerge from this cascade as a primary target for interventions that aim to interrupt the proposed cycle.