Abstract
Background
Since the introduction of trastuzumab into the treatment of Her-2/neu-positive metastatic breast cancer, cases of long-term survival have become more frequent. Even after tumor progression, trastuzumab seems to retain its antitumor activity which is potentiated by the combination with a chemotherapeutic agent.
Case Report
We are reporting about the unusual clinical course of a young patient with Her-2/neu-positive breast cancer, who experienced progression of pulmonary and bone metastases under treatment with trastuzumab. Upon progression, a combination therapy with capecitabine/trastuzumab was initiated, and a partial remission was achieved which has continued for over 4 years.
Conclusion
This unusual clinical course shows that continuing trastuzumab-based therapy beyond progression is a safe, effective, and well-tolerated option which can induce long-term remissions in some patients with Her-2/neu-positive metastatic breast cancer.
Keywords: Breast cancer, Metastasized, Capecitabine, Trastuzumab
Abstract
Hintergrund
Unter der Therapie mit dem monoklonalen Antikörper Trastuzumab beobachtet man nicht selten ein Langzeitüberleben bei Patientinnen mit metastasiertem, Her-2/neu-positiven Mammakarzinom. Auch nach der Tumorprogression scheint Trastuzumab eine antitumorale Wirkung beizubehalten, die durch die Kombination mit einer Chemotherapie wieder verstärkt werden kann.
Fallbericht
Wir berichten über einen ungewöhnlichen Fall einer jungen Frau mit metastasierter, Her-2/neu-positiver Brustkrebserkrankung und Zunahme der ossären und pulmonalen Metastasierung unter einer palliativen Trastuzumab-Therapie. Durch die Fortsetzung von Trastuzumab und der Kombination mit Capecitabin konnte wieder eine Partialremission erreicht werden, die seit über 4 Jahren anhält.
Schlussfolgerung
Dieser klinische Fall zeigt, dass Trastuzumab über die Progression hinaus eine sichere, effektive und gut tolerierte Therapieoption ist, mit welcher Langzeitremissionen bei bestimmten Patientinnen mit metastasiertem, Her-2/neu-positivem Mammakarzinom erzielt werden können.
Introduction
The introduction of trastuzumab (Herceptin®, Roche Pharma AG, Grenzach-Whylen, Germany) into the therapy of breast cancer has led to a significant improvement in survival for patients with Her-2/neu-positive disease [1]. Also in the metastatic setting, trastuzumab has been shown to improve response rates, time to disease progression, and overall survival when given in combination with chemotherapy or as monotherapy [2, 3], making trastuzumab an essential component of therapy for patients with Her-2/neu-positive breast cancer. Yet, the optimal treatment for patients who progress after or while undergoing treatment with trastuzumab is not well defined. In the past, options included stopping trastuzumab in favor of chemotherapy, or the more common clinical practice of continuing trastuzumab while changing its chemotherapeutic partner. This was based on empirical and retrospective data suggesting that trastuzumab retains its antitumor activity beyond progression [4, 5]. The first randomized trial to confirm these findings was the GBG-26 (trastuzumab beyond progression) study which randomized patients with metastatic Her-2/neu-positive breast cancer, who progressed under first-line trastuzumab-based therapies to capecitabine +/– trastuzumab. The study showed an improvement in response and time to progression for the trastuzumab-based combination therapy [6]. As newer therapies for Her-2/neu-positive breast cancer emerge (lapatinib, pertuzumab, TDM-1), the challenge of finding the optimal sequence and/or combination for individual patients will continue. The following clinical case is an impressive example of the antitumor activity of trastuzumab persisting beyond progression. It shows that continuing trastuzumab-based therapy is safe and well-tolerated and may induce prolonged remissions in selected patients.
Case Report
In June 2005, a 29-year-old female patient was found by her midwife to have a hard mass in her left breast a few days after delivering her second child. It was recommended that she stop breast feeding. Breast ultrasound and mammography supported the clinical suspicion of an inflammatory breast cancer measuring 11 cm with axillary lymph node involvement. A needle core biopsy confirmed the diagnosis, showing a poorly differentiated, invasive lobular breast cancer with negative hormone receptor status and overexpression of Her-2/neu. Staging examinations including chest X-ray, right upper quadrant ultrasound, and bone scan were performed, and ruled out distant metastases.
The decision was made to perform an anthracycline- and taxane-based preoperative chemotherapy. 6 cycles of docetaxel, adriamycin, and cyclophosphamide (TAC) were applied every 3 weeks according to the TAC protocol, achieving only minimal clinical tumor remission. A modified radical mastectomy and lymph node dissection were then performed, yielding 3 tumor nodules measuring 6.2, 2.0 and 1.5 cm of a poorly differentiated, invasive lobular breast cancer with minimal tumor regression (grade 0 according to Sinn). Resection margins were negative (R0 resection). 8 of 14 lymph nodes were involved with extracapsular extension, and there was presence of lymphatic and vascular invasion, as well as overexpression of Her-2/neu. Following the surgery, radiotherapy of the chest wall and infraclavicular lymph node region was performed.
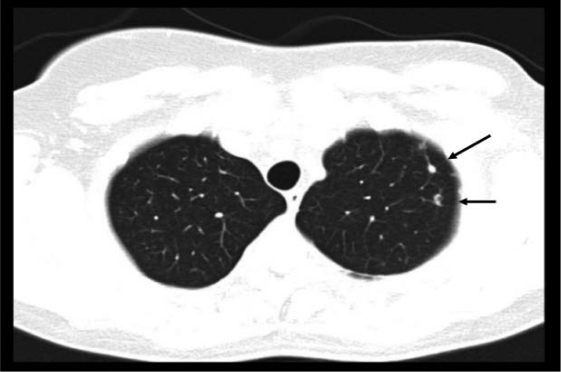
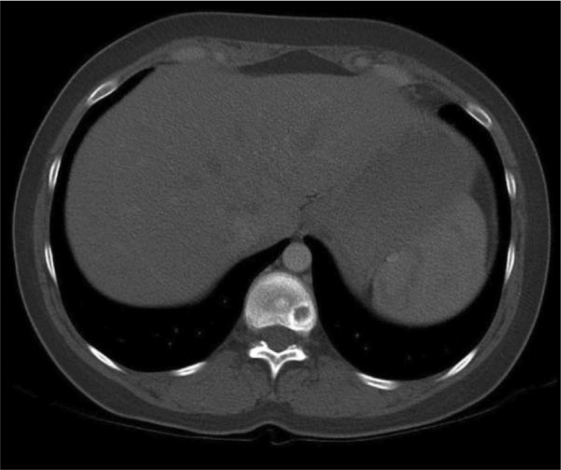
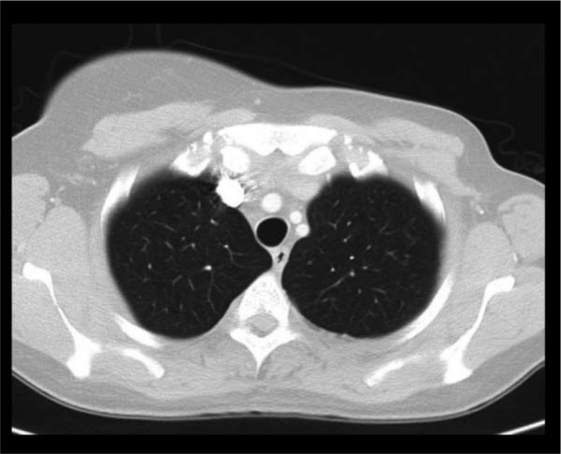
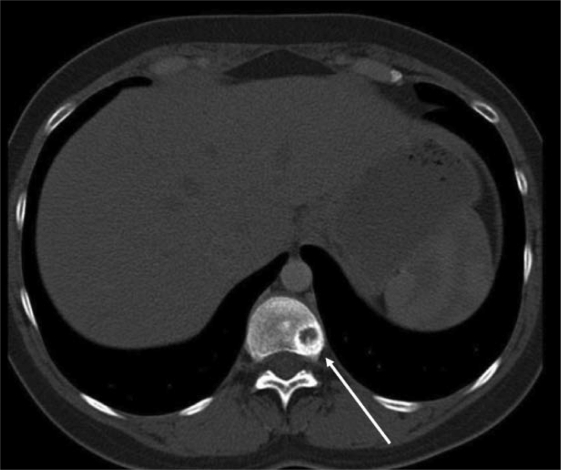
In November 2005, a computed tomography (CT) scan of the chest showed disseminated lung metastases and an osteolytic bone lesion in the 11th thoracic vertebra (T11). Hepatic lesions were not evident on ultrasound at this time. Due to the now metastatic stage of disease, therapy with the monoclonal antibody trastuzumab at a dose of 8 mg/kg followed by 6 mg/kg every 3 weeks was begun. In addition, she received the bisphosphonate ibandronate (Bondronat®, Roche) as a 6-mg intravenous infusion every 4 weeks. In May 2006, 6 months after starting on trastuzumab, a restaging chest CT showed evidence of progressive disease (figs. 1–2). Thus, it became necessary to offer new therapy options. At this time, our tumor center was participating in the GBG-26 study. In this study, patients progressing on trastuzumab for metastatic Her-2/neu-positive breast cancer were randomized to either oral capecitabine alone or in combination with trastuzumab. Our patient agreed to participate, and was randomized to the experimental arm with capecitabine plus trastuzumab, which she has been receiving in our outpatient chemotherapy unit since June 2006. The bisphosphonate therapy has also been continued. A chest CT performed in April 2007, revealed complete remission of her pulmonary metastases (fig. 3+4), which has been confirmed by repeated follow-up chest CTs, the latest in November 2010.
Fig. 1.
Computed tomography scan of the chest. Axial image of the upper lung fields showing 2 pulmonary metastases located subpleurally and measuring approximately 1 cm.
Fig. 2.
Computed tomography scan of the chest. Axial image of the thoracic spine showing an osteolytic lesion located posterolaterally on the left side of the vertebral body of T11.
Fig. 3.
Computed tomography scan of the chest. Axial image of the upper lung fields showing complete remission of pulmonary metastases in April 2007.
Fig. 4.
Computed tomography scan of the chest. Axial image of the thoracic spine showing a wide circular region of sclerosis around the outer edge of the osteolytic lesion, which is evidence for reparative changes under therapy.
The patient reports tolerating the ongoing therapy with capecitabine/trastuzumab/ibandronate well. Due to oncholysis and hand-foot syndrome (CTC grade 2), the capecitabine therapy had to be interrupted twice for a period of 6 weeks, and was once given at a 50% reduced dose for 3 weeks. The toxicities improved rapidly with these changes, and the therapy with the full dose of 2,500 mg/m2 days 1–14, 21 was resumed and continued. Under ongoing therapy, the patient is feeling well and is working part time. Repeated echocardiograms have been performed ruling out left ventricular dysfunction under trastuzumab. Furthermore, a reconstructive breast surgery has been performed with good cosmetic results. With careful dental hygiene and regular check-ups, no bisphosphonate complications (i.e. osteonecrosis of the jaw) have been observed so far.
Discussion
The long established paradigm that a chemotherapeutic agent should be stopped and a new agent begun when disease progression occurs was called into question in the case of trastuzumab and Her-2/neu-positive breast cancer, and may not apply to other targeted agents. Preclinical studies suggested that trastuzumab continues to have antitumor activity beyond progression in the treatment of Her-2/neu-positive metastatic breast cancer [7]. Several retrospective studies comparing outcomes for patients who progressed while receiving trastuzumab also showed that those who continued with trastuzumab-based therapies had significantly improved response rates, time to disease progression, and overall survival compared to those who stopped trastuzumab [4, 5]. In the GBG-26 study, the standard at the time (discontinuing trastuzumab and beginning a new chemotherapy with capecitabine) was compared with the experimental treatment (continuing trastuzumab in combination with the new chemotherapeutic agent, capecitabine). Because of insufficient recruitment, the study was closed after randomizing 156 patients. However, results showed a statistically significant improvement in progression-free survival and response rate for patients receiving trastuzumab combined with capecitabine, compared to those receiving capecitabine alone [6]. This randomized study provided proof of principle that trastuzumab continues to show antitumor activity beyond progression, and this activity is potentiated when combined with chemotherapy. The effect is believed to be mediated by antibody-dependant cellular cytotoxicity (ADCC): trastuzumab binds to Her-2/neu-positive tumor cells, natural killer cells then bind to the Fc region of trastuzumab and, upon binding, release their cytotoxic agents. The ADCC effect is potentiated by the addition of a new chemotherapeutic agent [8].
Conclusion
Our case is an impressive demonstration of the antitumor activity of trastuzumab beyond progression in metastatic breast cancer. Combining trastuzumab with capecitabine at the time of progression led to a partial remission of our patient's pulmonary and bone metastases, which is persisting for more than 4 years. Furthermore, the combined therapy is very well tolerated, with no signs of cumulative toxicity and only few reversible side effects. This clinical course is especially gratifying, since this young patient is able to work and take part in normal daily activities despite her advanced disease. As new therapeutic options for patients with progressive Her-2/neu-positive metastatic breast cancer emerge, the choice of therapy for individual patients is becoming more complex. Until the optimal combination or sequence of agents is established, trastuzumab-combined therapy remains a safe and effective option which has been shown to improve progression-free survival and may induce prolonged remission in selected patients.
Disclosure Statement
The authors did not provide a disclosure statement.
References
- 1.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marty M, Cognetti F, Maraninchi D, et al. Randomized ph II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2 positive metastatic breast cancer administered as first line treatment: the M770001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in the first line treatment of her2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Stemmler HJ, Kahlert S, Siekiera W, Untch M, et al. Prolonged survival of patients receiving trastuzumab beyond disease progression for her 2 overexpressing metastatic breast cancer. Onkologie. 2005;28(2):582–586. doi: 10.1159/000088296. [DOI] [PubMed] [Google Scholar]
- 5.Cancello G, Montagna E, Agostino D, et al. Continuing trastuzumab beyond disease progression: outcomes analysis in patients with metastatic breast cancer. Breast Cancer Res. 2008;10(2):R60. doi: 10.1186/bcr2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, Baumann KH, Clemens MR, Duerr R, Uleer C, Andersson M, Stein RC, Nekljudova V, Loibl S. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 7.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 8.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]