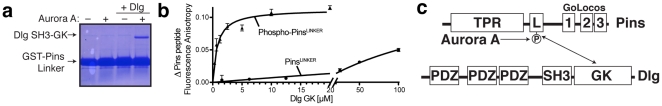
Figure 1. The Guanylate Kinase domain is an enzyme-derived phosphoprotein recognition domain.
(A) The Dlg GK domain is a specific phosphoprotein recognition domain. A GST-pull down experiment shows that the Dlg SH3-GK region only interacts with the Pins Linker domain when it has been phosphorylated by Aurora A. (B) Change in fluorescence anisotropy of phosphorylated and unphosphorylated Pins Linker peptides as a function of Dlg GK domain concentration. The curves represent binding affinities of 0.8 µM (phosphorylated) and 206 µM (unphosphorylated). (C) Domain structure of Pins and Dlg. Pins consists of Tetratricopeptide repeats (TPR), a linker domain (L), and three GoLoco motifs (1–3). Dlg contains three PDZ domains, and SH3 domain, and the GK domain. The mitotic kinase Aurora A phosphorylates the Pins Linker domain initiating an interaction with the Dlg GK domain.