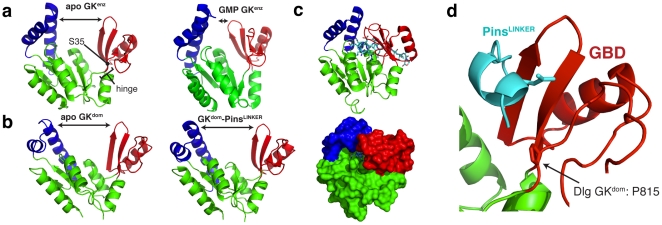
Figure 5. Loss of a ligand-induced conformational change in the GK domain.
(A) Nucleotide binding to the GK enzyme induces a large conformational change. GMP binding causes a transition from an “open" conformation to a “closed" one allowing for long range communication between the ATP and GMP binding sites. The “hinge" region that undergoes dihedral angle changes during closing is shown. Recently it was found that mutation of a serine residue (S35) in this region to proline is sufficient to convert enzyme to domain [29]. Structures 1EX6 and 1EX7 are shown [10]. (B) The GK domain conformation does not change upon ligand binding. Unlike the GK enzyme, the GK domain does not assume a “closed" conformation upon Pins binding to the GBD. (C) GK closing is incompatible with Pins binding to the GBD. Ribbon and surface representations are shown of Pins aligned to the closed GK enzyme structure (1EX7) showing that closing would cause dramatic steric overlaps with the protein ligand. (D) A critical serine to proline mutation is in the GK “hinge" region. Mutation of a conserved GK enzyme serine is sufficient to convert it into a GK domain [29]. The position of this proline, which lies in the region that undergoes dramatic dihedral angle changes in GK enzyme, is shown in a ribbon representation.