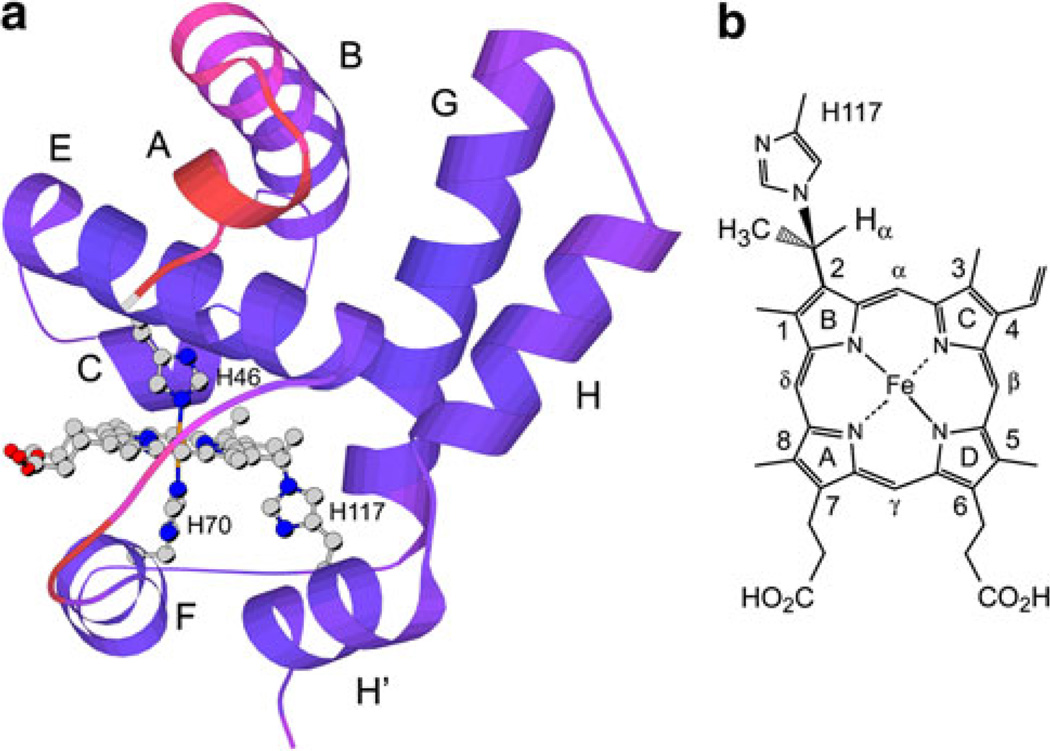
Fig. 1.
a Solution structure of the hemoglobin from Synechococcus sp. PCC 7002 with cross-linked heme (GlbN-A) in the ferric state [4]. A low-energy member of the 2KSC family is shown with redder colors indicating larger ensemble root mean square deviation from the average. Helices, axial histidines (His46, distal, and His70, proximal), and reactive histidine (His117) are labeled. b The structure of the His117–heme covalent linkage [7]