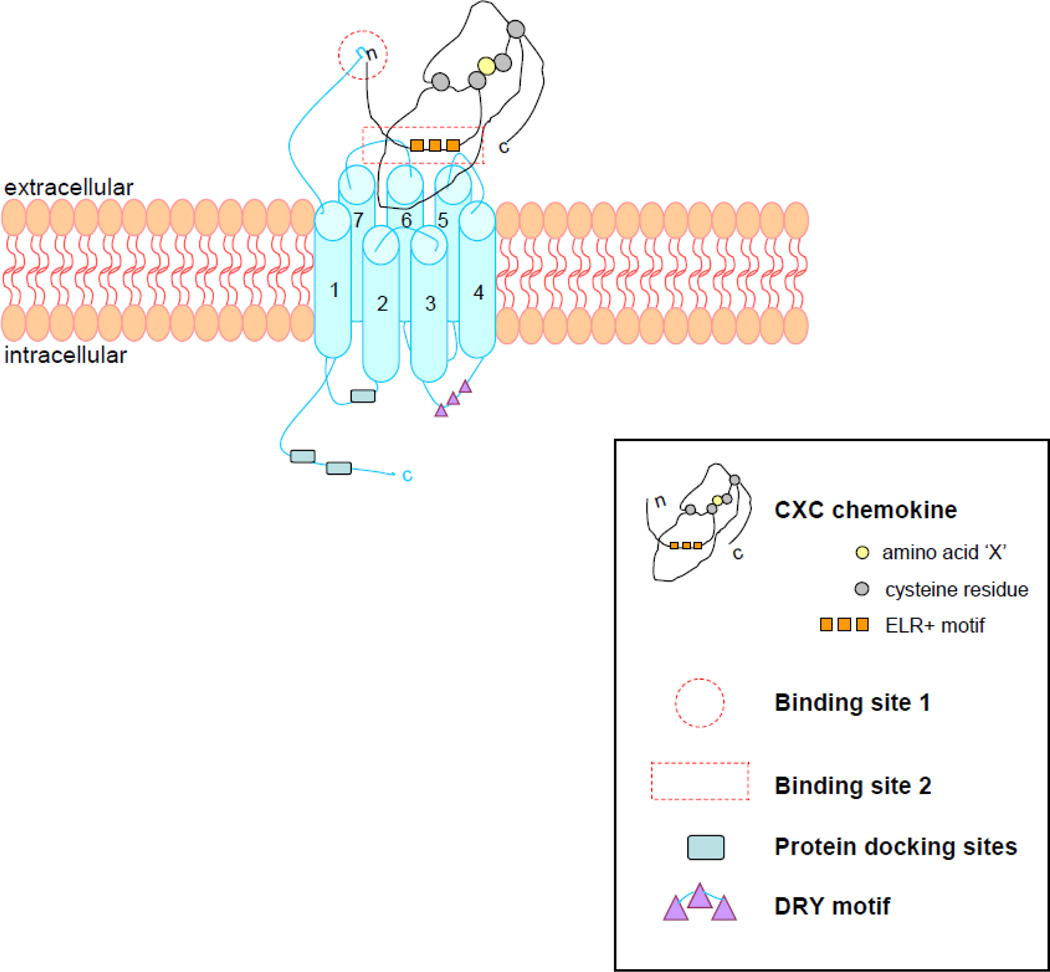
Figure 1. CXCR2 structure and ligand binding concept.
The CXCR2 chemokine receptor is compromised of seven transmembrane segments, three extracellular and three intracellular loops, an extracellular N-terminal domain, and an intracellular C-terminal domain. It contains a DRY motif on the second intracellular loop, functioning as G-protein docking site, and other protein docking sites as the LLKIL motif on the C-terminus.
The CXCR2 ligand N-terminus binds with the N-terminal domain of the receptor (site 1), after which the ELR+ motif of the chemokine binds with the second and third extracellular loop of the receptor (site 2), to control the binding and to activate receptors signaling pathways.