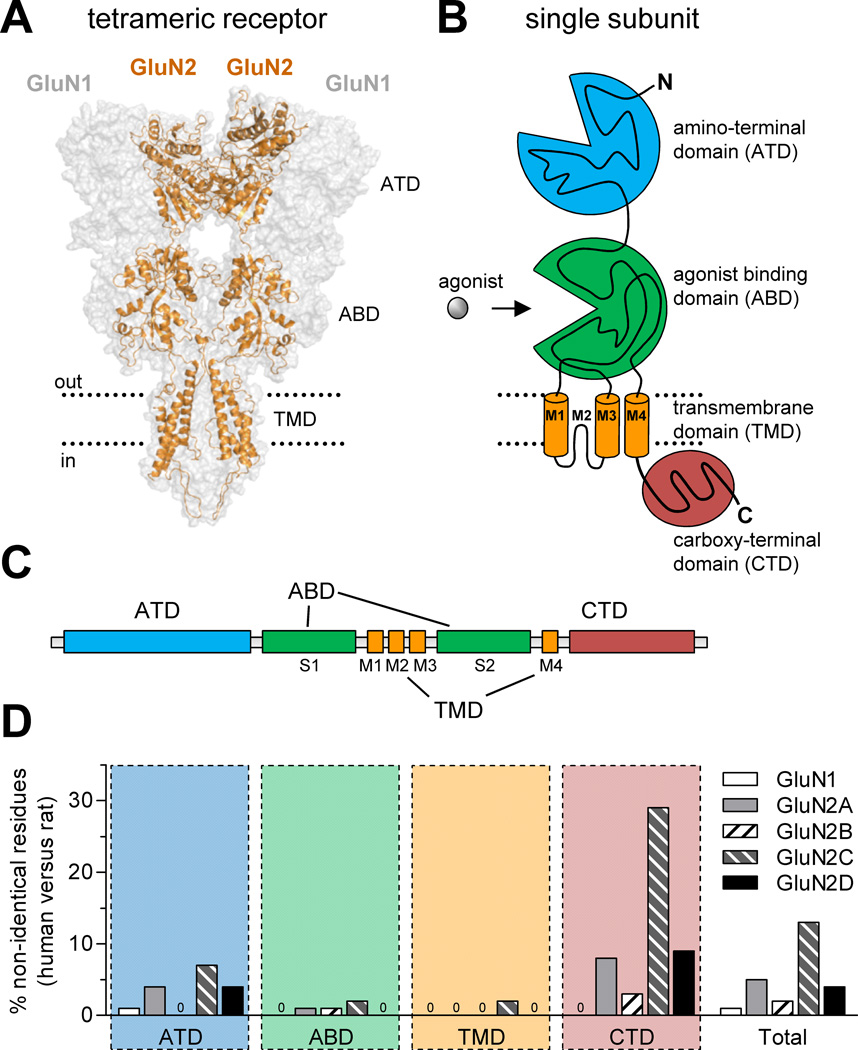
Figure 1.
Comparison of amino acid sequences for rat and human NMDA receptor subunits. (A) Model of the NMDA receptor based on the crystal structure of the membrane-spanning GluA2 AMPA receptor (Sobolevsky et al., 2009), showing the tetrameric arrangement of two GluN1 and two GluN2 subunits. The intracellular carboxyl-terminal domain (CTD) was not part of the crystal structure and is omitted from the model, which was generated as previously described (Acker et al., 2011). (B) NMDA receptor subunits consist of four semi-autonomous domains, namely the extracellular amino-terminal domain (ATD), the bi-lobed agonist binding domain (ABD), the transmembrane domain (composed of the transmembrane helices M1, M3, and M4, as well as the membrane re-entrant loop M2), and the intracellular carboxyl-terminal domain (CTD). (C) Linear representation of the polypeptide chain of NMDA receptor subunits, illustrating the relative positions of the four semi-autonomous domains in the amino acid sequence. The agonist binding domain (ABD) is formed by two amino acid segments termed S1 and S2. (D) The bar graph shows the percentage of residues that are not conserved between human and rat subunits for the four different domains and for the total protein. Insertion or deletions in human subunits compared to rat subunits are also categorized as non-identical positions. See Table 1 for more detail.