Abstract
Progressive accumulation of α-synuclein is key to the pathology of many neurodegenerative diseases, including Parkinson disease and dementia with Lewy bodies. Increased intracellular levels of α-synuclein may be caused by enhanced expression or alterations in protein degradation pathways. Here we review our recent study showing that the ubiquitin-proteasome system and the autophagy-lysosomal pathway are differentially involved in α-synuclein's degradation in vivo. We discuss the key findings obtained with our novel in vivo approach and also present a model for the progression of protein aggregation and dysfunctional degradation in Parkinson disease.
Key words: neurodegeneration, Parkinson disease, Lewy bodies, α-synuclein, degradation, autophagy, lysosome, proteasome, in vivo, multiphoton imaging
Alpha-synuclein is a key protein in the pathology of many neurodegenerative diseases, including Parkinson disease (PD) and dementia with Lewy bodies. While little is known about the physiological function of this protein, its importance for PD has been exemplified by genetic studies that showed point mutations can lead to rare familial forms of the disease, and by the seminal finding that Lewy bodies, the pathological hallmark of PD, largely contain phosphorylated and ubiquitinated forms of α-synuclein. The cascade by which α-synuclein misfolds, aggregates and causes havoc is not yet fully understood. One clear predisposing factor, however, is a frank increase in intracellular α-synuclein levels. Synthesis, post-translational modification, proper folding and removal through protein degradation pathways influence the cellular levels of any given protein. Enhanced expression of α-synuclein through gene multiplication or polymorphisms in its promoter can lead to familial forms or increased susceptibility to PD, respectively. In addition, genetic or viral vector-mediated overexpression can induce features of PD in a variety of animal models. Although important, abnormal expression of α-synuclein is only one side of the coin. Equally important to α-synuclein accumulation are alterations in protein degradation pathways that have been implicated by genetic, pathological and experimental studies. Earlier investigations have mainly focused on dysfunction of the ubiquitin-proteasome system (UPS), but a role for malfunction of the autophagylysosomal pathway (ALP) has been explored in recent years. The concept of dysfunctional degradation in PD is supported by studies in knockout models that show profound neurodegeneration and protein inclusion bodies in mice that lacked essential components of the ALP (Atg5 or Atg7) or the UPS (Psmc1). Crucial to our understanding of the role of degradation pathways in α-synuclein pathology is the simple question of how α-synuclein is degraded by neurons. Published studies, mainly in cell-culture models, have unfortunately provided conflicting results, allowing no definite conclusion. To understand α-synuclein's degradation at the in vivo level, we have combined topical application of inhibitors of protein degradation with in vivo multiphoton imaging to systematically test the role of the UPS and ALP in the living mouse brain.
The Results of our Study can be Summarized in Five Key Findings
(1) Alpha-synuclein is degraded by the UPS independent of the pre-existing α-synuclein burden, hence both in α-synuclein transgenic mice with pathologically elevated α-synuclein levels and in non-transgenic mice with only endogenous levels of this protein.
(2) The increase in α-synuclein following proteasome inhibition in α-synuclein transgenic mice occurs in an age-dependent manner, potentially indicating a reduced capacity of the UPS in aged animals.
(3) The ALP, and macroautophagy in particular, is recruited to degrade α-synuclein only in a pathological scenario with increased levels in α-synuclein-expressing transgenic mice.
(4) Using in vivo multiphoton imaging in α-synuclein-eGFP transgenic mice, we demonstrate that both somatic and pre-synaptic α-synuclein levels increase following either UPS or ALP inhibition.
(5) Both degradation pathways, the UPS and macroautophagy, are functionally coupled in α-synuclein transgenic mice such that inhibition of one leads to a compensatory upregulation of the other.
To explore the role of the UPS in vivo we used a modified cranial-window approach to apply the proteasome inhibitors clasto-lactacystin β-lactone (CLBL) and MG132. We demonstrate that these inhibitors can penetrate cortex and reliably block proteasome function up to ∼600 mm deep in the brain. Confirming previous reports, we found that α-synuclein transgenic mice display significantly lower proteasome activity than their non-transgenic littermates at baseline. We also discovered that aged α-synuclein transgenic mice are more vulnerable to proteasome inhibition and thus accumulate more α-synuclein after CLBL application.
Turning to autophagy, we found that topical application of bafilomycin A1 (BafA1) impairs the ALP in the first 300 µm of cortex, as measured by immunoblot levels of both LC3-II and p62, a known substrate of macroautophagy. In contrast to UPS inhibition, however, ALP inhibition increases α-synuclein levels only in α-synuclein transgenic mice and not in wild-type controls. This leads us to hypothesize that autophagy is involved in α-synuclein degradation only in a pathological scenario where increased α-synuclein burden exists. Our in vivo multiphoton imaging experiments of asynuclein-eGFP transgenic mouse brain support this finding. Both UPS and ALP inhibition leads to significantly increased levels of α-synuclein-eGFP fluorescence signal in the soma and presynaptic terminals, arguing for an accumulation of the fusion protein in these subcellular compartments.
Because these data suggest distinct roles for the UPS and ALP in the degradation of α-synuclein in vivo, we next investigated potential crosstalk between both pathways. We detected elevated levels of the autophagosome marker LC3-II and a reduction of p62 in animals that were treated with the proteasome inhibitor CLBL. In the opposite direction, catalytic proteasome activity is increased in tissue recovered from animals treated with the autophagy inhibitor BafA1. Importantly, these apparent compensatory actions are only present in α-synuclein transgenic mice, arguing that the intracellular level of aggregate prone proteins can modulate a potential crosstalk between degradation pathways. Although these findings are descriptive, the interplay between degradation pathways is an interesting question for future research, because learning about the cellular response to protein accumulation can improve understanding of disease progression and help in developing strategies to counteract protein accumulation in a stage-adapted manner (Fig. 1).
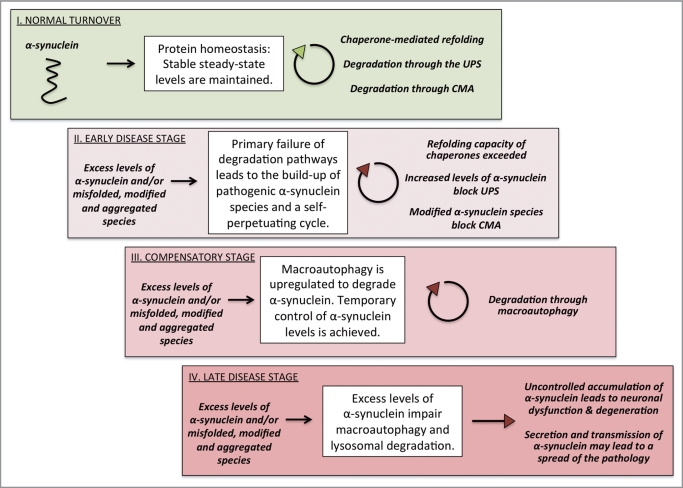
Figure 1.
Proposed model for the role of protein degradation pathways in Parkinson disease. Primary dysfunction of protein degradation may lead to excess levels of α-synuclein and the formation of pathologically modified species that overwhelm the capacity of chaperones, the ubiquitin-proteasome system (UPS) and chaperone-mediated autophagy (CMA). In the next stage, macroautophagy is induced and temporary homeostasis is achieved before this balance finally tilts. Entering the late disease stage, α-synuclein inhibits macroautophagy and lysosomal degradation. Uncontrolled accumulation produces oligomeric species that cause neuronal dysfunction and degeneration. At the same time, excess levels of α-synuclein might trigger α-synuclein secretion, thus potentially contributing to a spread of the pathology.
Summa summarum, our study demonstrates distinct roles for the UPS and autophagy in the degradation of α-synuclein in vivo and emphasizes collaborative actions. Our results indicate a link between degradation pathways and α-synuclein pathology by providing evidence for a relationship between the protein burden and the mechanism recruited to maintain protein homeostasis. In conjunction with previous reports, a disease model for the progression of protein aggregation and dysfunctional degradation can be envisioned (Fig. 1). A more advanced understanding of the degradation pathways in PD will lead to rational drug design and hopefully future therapeutics that target degradation pathways to counteract α-synuclein pathology.
Acknowledgment
We are grateful for funding from the NIH (P.J.M., V.K.U.) and fellowships from the German National Academic Foundation (D.E.-F.), the Hamburg Foundation for International Research and Studies (D.E.-F.) and the Parkinson's Disease Foundation (D.E.-F.).
Abbreviations
- ALP
autophagy-lysosomal pathway
- BafA1
bafilomycin A1
- CLBL
clasto-lactacystin beta-lactone
- CMA
chaperone-mediated autophagy
- eGFP
enhanced green fluorescent protein
- PD
Parkinson disease
- UPS
ubiquitinproteasome system
Punctum to: Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, et al. Distinct roles in vivo for the ubiquitin proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci. 2011;31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011.