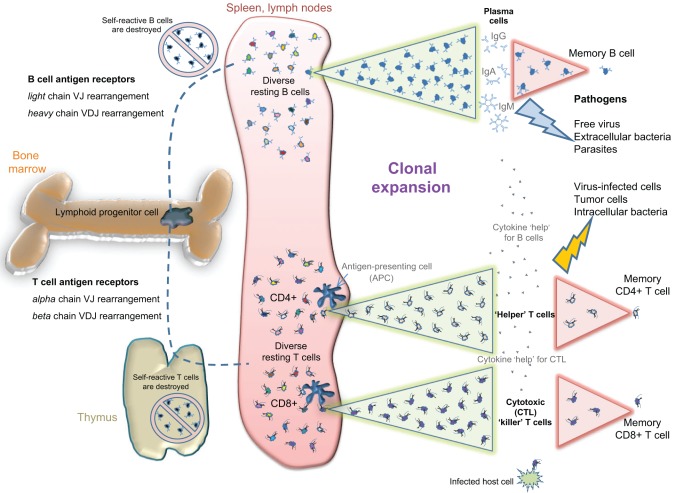
Fig. 1.
Vertebrate adaptive immunity. Antigen diversity in jawed vertebrates is generated by somatic recombination of the V, D and J regions (variable, diversity and joining immunoglobulin gene regions) in both immunoglobulin (Ig, B-cell receptor) and TCR (T-cell receptor) chains. After their development in bone marrow (and thymus for T cells), the clones that express self-reactive antigen receptors are deleted. Very few mature resting B and T cells are maintained for each specific antigen (indicated with different colors), thus conserving valuable metabolic resources. Upon antigen exposure in the periphery, the appropriate lymphocyte clone(s) are expanded quickly through rapid proliferation to generate large numbers of antigen-specific effector cells (clonal expansion, illustrated by the green triangles). Effector B cells secrete soluble receptors (antibodies, i.e. IgG, IgA and IgM) that can neutralize or eliminate extracellular pathogens. Cytotoxic (CD8+) T cells scan host cells for the presence of virus or intracellular bacteria, killing any cells that are infected. Helper (CD4+) T cells secrete cytokines that boost both cytotoxic T cells and effector B cells for efficient class-switched antibody responses. Most effector cells die after pathogen clearance, leaving only small numbers of memory B and T cells that are poised for a more rapid clonal expansion upon a secondary exposure (illustrated by the pink triangles). Thus, adaptive immunity utilizes clonal expansion to provide long-lasting specific protection against a wide variety of pathogens while minimizing nutrient cost to the host. IgG, immunoglobulin γ; IgA, immunoglobulin α; IgM, immunoglobulin μ.