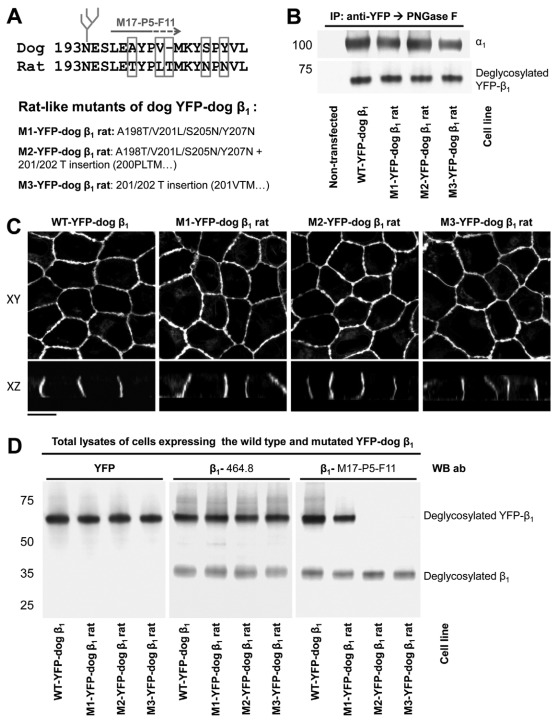
Fig. 3.
Rat-like mutations in the amino acid region 198–207 of YFP–dog-β1 impair the recognition of YFP–dog-β1 by an adhesion-blocking M17-P5-F11 antibody. (A) The positions of the amino acid residues mutated in the amino acid region 198–207 of YFP–dog-β1 are shown. Three mutants were constructed, the first with four rat-like amino acid substitutions, the second with four substitutions and one amino acid insertion, and the third with the amino acid insertion only. (B) Western blot analysis of the proteins precipitated by an antibody against YFP shows co-precipitation of the endogenous α1 subunit with the wild-type and mutated YFP–dog-β1 fusion proteins. (C) Confocal microscopy images of stable cell lines expressing the wild-type and mutated YFP–dog-α1 fusion proteins show their predominant localization in the lateral membranes. All images were taken with the same settings. Scale bar: 10 μm. (D) Equal amounts of total cell lysates of MDCK cells expressing either wild-type or mutated YFP–dog-β1 were analyzed by immunoblotting using antibodies to YFP and two types of monoclonal antibodies to β1. Mutations in the amino acid region 198–207 did not have an effect on the reactivity of the 464.1 anti-β1 antibody with YFP–dog-β1. By contrast, replacement of four residues in the amino acid region 198–207 resulted in a substantial decrease in the reactivity of the M17-P5-F11 antibody with YFP–dog-β1. Insertion of a rat-unique Thr residue resulted in a complete loss of reactivity of the M17-P5-F11 antibody with YFP–dog-β1. Here and below, to enable quantitative comparison, immunoprecipitated proteins were deglycosylated by PNGase F prior to SDS-PAGE.