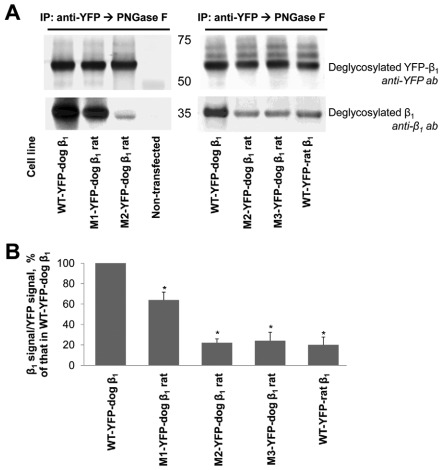
Fig. 4.
Rat-like mutations in the amino acid region 198–207 of the YFP–dog-β1 impair (YFP–β1)–β1 interactions. (A) Western blot analysis using antibodies against YFP and Na+/K+-ATPase β1 shows that immunoprecipitation of YFP–β1 resulted in co-precipitation of the endogenous Na+/K+-ATPase β1 subunits from cell lysates of MDCK cells expressing the wild-type YFP–dog-β1, or its mutants or the wild-type YFP–rat-β1. (B) Densitometric quantification of the results presented in A was performed by dividing a signal from the antibody to β1 by the corresponding signal from the antibody to YFP. The comparative bar graph shows these ratios as a percentage of the ratio obtained for the wild-type YFP–β1. The results of quantification indicate that amino acid substitutions and insertion in the amino acid region 198–207 decreased the amount of YFP–β1-co-immunoprecipitated endogenous β1 subunits. The positions of the amino acid residues mutated are shown in Fig. 3A. WT, wild-type; error bars, ±s.d. (n=3); *, significant difference from the ‘WT-YFP–dog-β1’, P<0.01, Student's t-test.