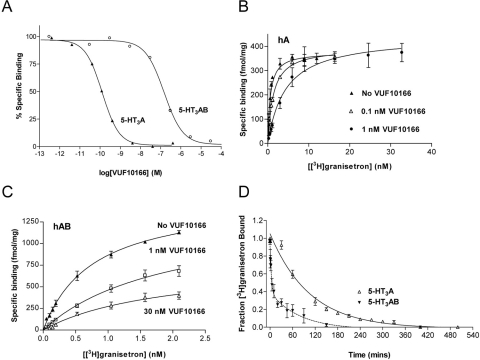
Fig. 2.
Binding properties of VUF10166 at 5-HT3A and 5-HT3AB receptors expressed in HEK293 cells. A, typical experiments showing that the displacement of specific [3H]granisetron binding by VUF10166 was different (p > 0.05) at 5-HT3A and 5-HT3AB receptors. B, [3H]granisetron binding curves for wild-type 5-HT3A receptors (Kd = 0.78 ± 0.16 nM, Bmax = 382 ± 18 fmol/mg, n = 3) in the presence of 0.1 nM VUF10166 (Kd = 1.83 ± 0.30 nM*, Bmax = 413 ± 21 fmol/mg, n = 3) and 1 nM VUF10166 (Kd = 4.32 ± 1.00 nM*, Bmax = 443 ± 32 fmol/mg, n = 4). C, [3H]granisetron binding curves for wild-type 5-HT3AB receptors (Kd = 0.77 ± 0.05 nM, Bmax = 1529 ± 40 fmol/mg, n = 4) and in the presence of 1 nM VUF10166 (Kd = 2.63 ± 0.96 nM, Bmax = 1272 ± 392 fmol/mg, n = 3) and 30 nM (Kd = 1.89 ± 0.57 nM, Bmax = 789 ± 135 fmol/mg*, n = 4). These concentrations are greater than or equal to the affinities of VUF10166 at 5-HT3A (Ki = 0.04 nM) and 5-HT3AB (Ki = 22.4 nM) receptors. D, dissociation of [3H]granisetron after the addition of excess (100 μM) unlabeled VUF10166. Dissociation at the 5-HT3A receptor was best fit with a single exponential (t1/2 = 53.3 min), and 5-HT3AB receptors were best fit with a double exponential (t1/2 = 2.33 and 55.1 min). Sample sizes and rate constants can be found in the text. Values are mean ± S.E.M. *, significantly different from values for the wild type (p < 0.05).