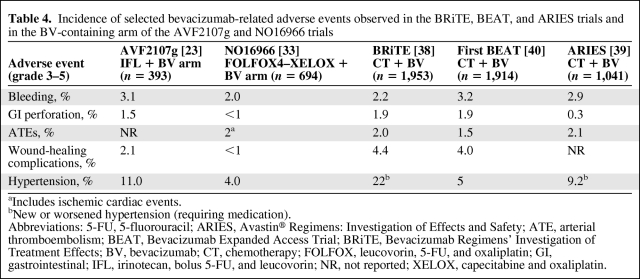
Table 4.
Incidence of selected bevacizumab-related adverse events observed in the BRiTE, BEAT, and ARIES trials and in the BV-containing arm of the AVF2107g and NO16966 trials
aIncludes ischemic cardiac events.
bNew or worsened hypertension (requiring medication).
Abbreviations: 5-FU, 5-fluorouracil; ARIES, Avastin® Regimens: Investigation of Effects and Safety; ATE, arterial thromboembolism; BEAT, Bevacizumab Expanded Access Trial; BRiTE, Bevacizumab Regimens' Investigation of Treatment Effects; BV, bevacizumab; CT, chemotherapy; FOLFOX, leucovorin, 5-FU, and oxaliplatin; GI, gastrointestinal; IFL, irinotecan, bolus 5-FU, and leucovorin; NR, not reported; XELOX, capecitabine and oxaliplatin.