Abstract
Background and Aims
Foliar variegation is recognized as arising from two major mechanisms: leaf structure and pigment-related variegation. Begonia has species with a variety of natural foliar variegation patterns, providing diverse examples of this phenomenon. The aims of this work are to elucidate the mechanisms underlying different foliar variegation patterns in Begonia and to determine their physiological consequences.
Methods
Six species and one cultivar of Begonia were investigated. Light and electron microscopy revealed the leaf structure and ultrastructure of chloroplasts in green and light areas of variegated leaves. Maximum quantum yields of photosystem II were measured by chlorophyll fluorescence. Comparison with a cultivar of Ficus revealed key features distinguishing variegation mechanisms.
Key Results
Intercellular space above the chlorenchyma is the mechanism of variegation in these Begonia. This intercellular space can be located (a) below the adaxial epidermis or (b) below the adaxial water storage tissue (the first report for any taxa), creating light areas on a leaf. In addition, chlorenchyma cell shape and chloroplast distribution within chlorenchyma cells differ between light and green areas. Chloroplasts from both areas showed dense stacking of grana and stroma thylakoid membranes. The maximum quantum yield did not differ significantly between these areas, suggesting minimal loss of function with variegation. However, the absence of chloroplasts in light areas of leaves in the Ficus cultivar led to an extremely low quantum yield.
Conclusions
Variegation in these Begonia is structural, where light areas are created by internal reflection between air spaces and cells in a leaf. Two forms of air space structural variegation occur, distinguished by the location of the air spaces. Both forms may have a common origin in development where dermal tissue becomes loosely connected to mesophyll. Photosynthetic functioning is retained in light areas, and these areas do not include primary veins, potentially limiting the costs of variegation.
Keywords: Begonia, chlorenchyma, chlorophyll fluorescence, chloroplast, Ficus pumila ‘Sonny’, intercellular space, internal reflection, ultrastructure, variegation
INTRODUCTION
Variegated leaves are defined by the presence of multiple colours on the leaf surface variously arranged as irregular spots or patches, and regular patterns. Variegated plants are popular as ornamentals, and include various species and cultivars of Aglaonema, Begonia, Coleus blumei, Codiaeum variegatum, Cyclamen and Saxifraga stolonifera. Although rare in nature, in tropical and sub-tropical environments, variegated leaves are relatively commonly found in forest understoreys on juveniles of trees and shrubs and on shade plants including ferns (e.g. Pteris), gymnosperms (e.g. Podocarpus madagascariensis) and flowering plants (e.g. Begoniaceae, Melastomataceae and Myrsinaceae) (C.-R. Sheue, pers. obs.). The adaptive significance of foliar variegation is not well understood (Tsukaya et al., 2004). However, in Hydrophyllum virginianum, leaf variegation has been associated with reduced herbivore damage (Campitelli et al., 2008).
In a classic work based on a study of 55 species from 24 families, Hara (1957) identified four mechanisms of foliar variegation, which he named ‘chlorophyll type’, ‘pigment type’, ‘air space type’ and ‘epidermis type’. These mechanisms fall into two groups: pigment-related variegation (chlorophyll and pigment) and structural variegation (air space and epidermis) depending on how light areas of a leaf are created. For the chlorophyll type, light areas are caused by deficiency of chlorophyll (e.g. Crocus vernus and Saururus chinensis; Hara, 1957). For the pigment type, pigments other than chlorophyll are present, as in Coleus blumei (Fisher, 1986), Polygonum (Hara, 1957) and Tricyrtis (Hara, 1957). Structural variegation, however, does not involve variation in pigmentation. Most structural variegation observed by Hara (1957) was caused by diffuse reflection of light from air spaces just beneath the epidermis (the air space type), but in Oxalis martiana structural variegation was caused by variation in epidermal cell thickness.
Despite this important work of Hara (1957), many authors have assumed that variegation is due to chlorophyll deficiency alone, and variegation is sometimes reported without correct identification of the mechanism, often incorrectly stating that it must be due to pigments or plastids. Many studies have focused on variegation due to chlorophyll deficiency (Fisher, 1986; Aluru et al., 2001; Jiang et al., 2004), with only a few studies on structural variegation (Fooshee and Henny, 1990; Tsukaya et al., 2004).
Hara's classic 1957 work was done at a time well before modern microscopy. Moreover, the more recent studies mentioned above do not include information on chloroplast ultrastructure and photosynthetic performance. Here we report a study of novel variegated leaves of Begonia with various patterns. In order to understand the mechanism of variegation and the photosynthetic capacity of variegated leaves, leaf optical features, leaf anatomy and chloroplast ultrastructure were examined, and chlorophyll fluorescence was measured. Begonia is a large genus comprising >1500 named species (de Wilde, 2011). These plants display an amazing variety of shapes, colours, patterns and textures in their leaves rarely seen in other groups of plants (Kiew, 2005). Uneven distributions of pigmentation and silvery spots are commonly seen in the striking patterns of their naturally occurring variegated leaves (Kiew, 2005). Thus, they make ideal materials for better understanding the phenomena of natural foliar variegation. As the Begonia that we studied were all found to have structural variegation, we also examined a cultivar of Ficus with pigment type variegation to provide a comparison between naturally occurring structural variegation and the kind of variegation often occurring artificially in cultivars.
MATERIALS AND METHODS
Plant materials
We studied variegated leaves from six species and one cultivar of Begonia (Begoniaceae) and one Ficus cultivar (Moraceae) (Fig. 1). These six Begonia species were B. chlorosticta Sands, B. diadema Linden, B. formosana (Hayata) Masamune, B. hemsleyana Hook. f., B. pustulata Liebm. and B. versicolor Irmsch. (collected from the greenhouse of Academia Sinica in Taipei, Taiwan). The Begonia cultivar is an ornamental hybrid of Begonia designated ‘K030960’ at the Dr Cecelia Koo Botanic Conservation Center in Pingtung County, Taiwan, where the study material was obtained. The ornamental plant, F. pumila ‘Sonny’ was bought from a market in Taichung, Taiwan. Voucher specimens of all taxa except the cultivars were deposited in the HAST herbarium of Academia Sinica in Taipei.
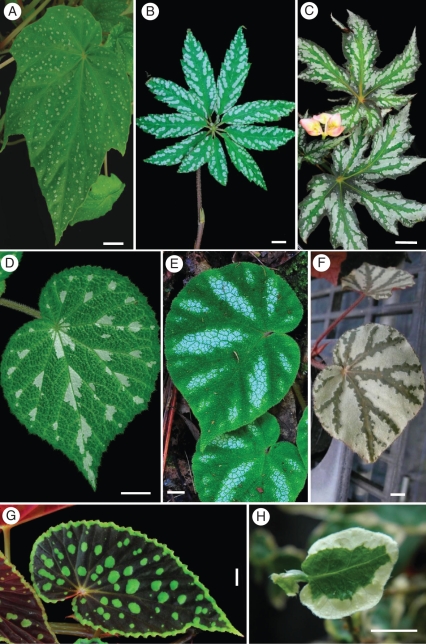
Fig. 1.
Various patterns of variegated leaves of (A–G) the seven taxa of Begonia studied and (H) Ficus pumila ‘Sonny’. (A) A spotted pattern consisting of large spots intermingled with tiny sand-like spots on leaves of B. formosana. (B and C) Chains of spots composed of spots and adjacent spots that run together on leaves of B. hemsleyana (B) and B. diadema (C). (D–F) White patches between the primary veins. (D) Begonia pustulata with small patches near the joint of two primary veins. (E) Begonia versicolor with patches not including secondary veins. (F) Begonia cultivar showing silvery areas between primary veins, contrasting strongly with the green veins. (G) Begonia chlorosticta showing striking large spots and a margin of light green on a dark green leaf background and red abaxial surface. (H) Ficus pumila ‘Sonny’ has broad white areas near and along the leaf margin on both sides. In contrast, the light areas of the Begonia leaves only appear on the adaxial surface (all scale bars = 1 cm).
Optical properties of the leaf
The adaxial surfaces of fresh leaves were observed with both transmitted and reflected light with a LEICA S8AP0 stereoscope (Wetzlar, Germany) equipped with an Olympus digital camera (Tokyo, Japan). Four Begonia (B. diadema, B. formosana, B. hemsleyana and B. pustulata) and F. pumila ‘Sonny’ were selected for these observations. Variegated leaves in these taxa have patterns made by white or light green areas (here called ‘light’) on a normal green coloured background (here called ‘green’).
Adaxial epidermal cell surface features
Both light and green areas of B. chlorosticta, B. diadema, B. formosana, B. pustulata and F. pumila ‘Sonny’ were cut to approx. 1 cm × 1 cm, placed on a cold stage (pre-frozen by liquid nitrogen) for 1–2 min and observed with a scanning electron microscope (TM3000 Tabletop Microscope, Hitachi, Tokyo, Japan).
Leaf structure and chloroplast ultrastructure
For all taxa, small pieces of leaf (1·0 × 1·0 mm2) from both light and green areas were cut and fixed in 2·5 % glutaraldehyde in 0·1 m sodium phosphate buffer (pH 7·3) overnight at 4 °C. These specimens were post-fixed in 1 % OsO4 in the same buffer for 4 h. After dehydration through an ethanol series, these materials were embedded in Spurr's resin (DER = 6·0) (Spurr, 1969). The embedded materials were then polymerized at 70 °C for 12 h. They were then cut into semi-thin sections (1·5 µm) with an MTX Ultramicrotome (RMC, Tucson, AZ, USA), and stained with 1 % toluidine blue for 2 min before observation with a light microscope (OLYMPUS BX-51, Tokyo, Japan). Ultrathin sections (70 nm) were cut and stained with 5 % uranyl acetate (in 50 % methanol) and 1 % lead citrate (in water) before examination with a transmission electron microscope (JEOL JEM-1400, Tokyo, Japan). In addition, freehand sections of a fresh leaf of B. chlorosticta were made to determine the location of red coloration.
Chlorophyll fluorescence measurement and analysis
The chlorophyll fluorescence of the light and green areas of four taxa (B. diadema, B. formosana, B. pustulata and F. pumila ‘Sonny’) was measured on intact growing plants to compare their photosynthetic performance. A pair of light and green areas of the same leaf, from each of five leaves, from an individual of each taxon, was selected for study. A pulse-modulated fluorescence system (FMS1) (Hansatech Instruments Ltd, Norfolk, UK) was used to measure photosystem II (PSII) quantum efficiency. The chlorophyll fluorescence parameters were measured after the leaves had been dark adapted for 20 min (Krause and Weis, 1984). The minimum fluorescence (Fo), with all PSII reaction centres open, was determined with a weak non-actinic modulated light (<0·1 µmol m−2 s−1). The maximum fluorescence (Fm), with all PSII reaction centres closed, was induced by a saturating pulse of white light (1 s duration, 13 000 µmol m−2 s−1) and measured after actinic light (300 µmol m−2 s−1) was applied. The variable fluorescence (Fv) was calculated as Fm – Fo. The ratio of variable to maximum fluorescence (Fv/Fm) represented the PSII quantum efficiency. For each taxon, differences in the Fv/Fm values between light and green areas were analysed with a paired t-test (Gauss 11, Aptech Systems Inc., Seattle, WA, USA). Residuals were examined for departure from normality to validate use of this test.
RESULTS
Leaf variegated patterns and optical features
The variegated leaves of the Begonia studied display three patterns of light areas on their adaxial surfaces: spots (B. formosana and B. chlorosticta), blotches (B. diadema and B. hemsleyana) and patches (B. pustulata, B. versicolor and the Begonia cultivar) (Fig. 1). Most light areas are white or silvery white, but in B. chlorosticta they are light green. Three species that have light spots either isolated (B. formosana) or in chains (B. diadema and B. hemsleyana) have a trichome in the centre of each spot. The white patches of B. pustulata usually occur near the junction of two primary veins (Fig. 1D), while those of B. versicolor are located in the areas between the primary veins, and all veins remain green (Fig. 1E). The adaxial leaf surface of the Begonia cultivar appears silvery between primary veins, contrasting strongly with the green veins of the leaf (Fig. 1F). The variegated leaves of B. chlorosticta are colourful, with striking large light green spots on a dark green background. The leaf margin is light green on the adaxial surface, and the entire abaxial surface is red (Fig. 1G).
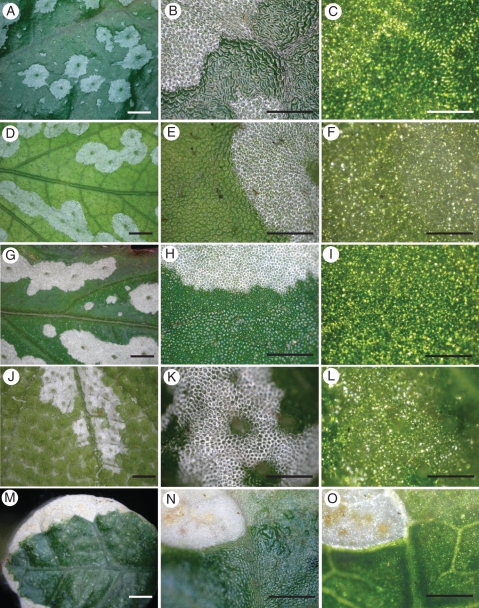
Variegated leaves of Begonia were observed using a stereomicroscope (Fig. 2). The light areas reflected much whiter light than the green areas (Fig. 2B, E, H, K). Under reflected light, in the light areas, each adaxial epidermal cell showed a striking irregular white ring outlining the green interior of the cell. Zhang et al. (2009) reported a similar phenomenon in B. rex, which they described as a polygonal pattern (PP). Here we found that this PP disappeared under transmitted light. In green areas of a leaf, only very thin faint white outlines of adaxial epidermal cells were visible under reflected light. Both light and green areas of a leaf showed very similar green colour with transmitted light, revealing similar pigments interior to a leaf beneath each area (Figs. 2C, F, I, L).
Fig. 2.
Adaxial surface patterns of variegated leaves of Begonia and Ficus pumila ‘Sonny’ observed with reflected light (first column, low magnification; second column, high magnification) and transmitted light (third column, image matched with the second column). (A–C) Begonia formosana. (D–F) Begonia diadema. (G–I) Begonia hemsleyana. (J–L) Begonia pustulata. (M–O) Ficus pumila ‘Sonny’. The fading of the variegation patterns of Begonia under transmitted light reveals their structural origin, in contrast to the absence of any change in the Ficus pattern. Also note that each adaxial epidermal cell in the light areas shows an irregular white ring (a polygonal pattern) under reflected light, outlining the cell, but this pattern disappears under transmitted light. Scale bars = 2 mm in the first column, 0·5 mm in the second and third columns.
Comparative morphology and anatomy
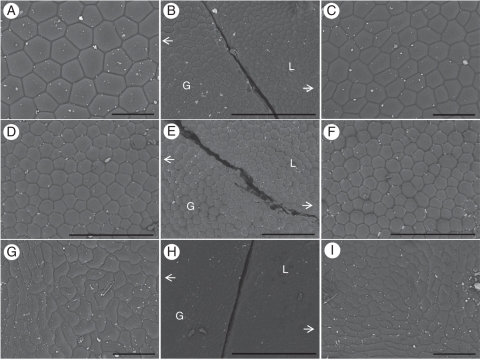
Epidermal cell morphology differs between the light and green areas of the adaxial epidermis in some Begonia. Adaxial epidermal cells of the light areas are smaller than those in the green areas of B. diadema and B. formosana (Fig. 3A–C). However, no differences in cell size or cell shape were evident between the two areas in B. chlorosticta (Figs. 3D–F) and B. pustulata.
Fig. 3.
Surface views of adaxial epidermal cells of variegated leaves of Begonia (A–C, B. formosana; D–F, B. chlorosticta) and F. pumila ‘Sonny’ (G–I). The second column with lower magnification shows a boundary marked by a knife cut between a green area (G) and a light area (L) of a variegated leaf. The first column and the third column show these same green and light areas, respectively, at the same higher magnification. Scale bars: (A, C, G, I) = 100 µm; (B, D–F, H) = 500 µm.
Like most eudicot species, the Begonia studied have dorsiventral leaves (Fig. 4A–C). These Begonia have funnel-shaped chlorenchyma, rather than the typical palisade leaf chlorenchyma, adjacent to the adaxial epidermis and spongy tissue connected to the abaxial epidermis. The Begonia cultivar differs from the other Begonia studied in having water storage tissue, which occurs in layers adjacent to each epidermal surface (Fig. 4D).
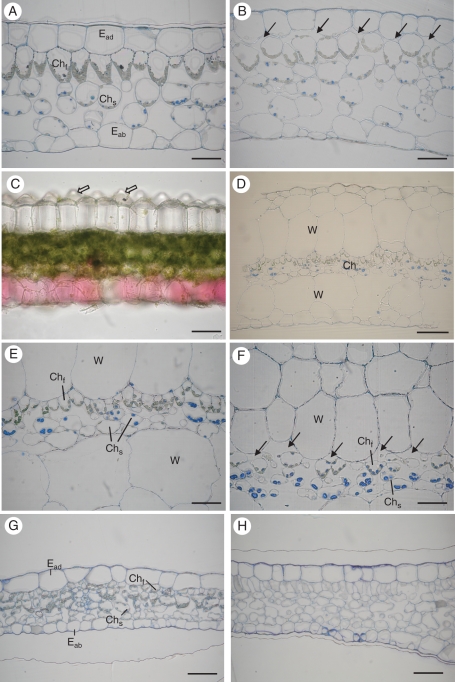
Fig. 4.
Transverse sections of variegated leaves from green (left) and light (right) areas of Begonia and Ficus pumila ‘Sonny.’ (A, B) Leaf of B. formosana in a green area and a light area (arrows indicate intercellular spaces). (C) Freehand section of B. chlorosticta showing the lens-like adaxial epidermis with conical protrusions (open arrows), and red coloration in the abaxial epidermis. (D–F) Leaf of the Begonia cultivar (D), and close-up views of the arrangement of water storage tissue and funnel-shaped chlorenchyma in a green area (E) and a light area (F, arrows indicate intercellular spaces). Note that the funnel-shaped chlorenchyma cells are in tight contact with the adaxial epidermis in green areas in Begonia, but the light areas of these leaves have intercellular spaces between the adaxial epidermis (or the water storage tissue) and the funnel-shaped chlorenchyma, with only occasional small spots of direct contact. (G, H) Ficus pumila ‘Sonny’. Chlorenchyma tissue in a green area (G) filled with chloroplasts. A light area revealing the absence of chloroplasts (H). Abbreviations: Eab, abaxial epidermis; Ead, adaxial epidermis; Chf, funnel-shaped chlorenchyma; Chs, spongy chlorenchyma; W, water storage tissue. Scale bars (A–C, E–H) = 50 µm; (D) = 100 µm.
With the exception of the Begonia cultivar, in the green areas of the Begonia leaves studied, the funnel-shaped chlorenchyma cells were in tight contact with the adaxial epidermis (Fig. 4A, C–E). In the light areas of these leaves, the intercellular space was commonly found between the adaxial epidermis and the funnel-shaped chlorenchyma (Fig. 4B). In light areas of Begonia cultivar leaves, similar intercellular spaces were evident, but between the funnel-shaped chlorenchyma and the water storage tissue (Fig. 4F). The same leaf anatomy defines light and green areas of B. chlorosticta (Fig. 4C). Its red coloration was found in the abaxial epidermis, and was present in all areas of a leaf.
The intercellular space, however, was not the only anatomical distinction between light and green areas of leaves in the Begonia of this study: chlorenchyma cell shapes and chloroplast locations also differed. In green areas, the chloroplasts of the funnel-shaped chlorenchyma are confined to the tapering lower part of the cell, lining the cell wall. Unlike the typical funnel-shaped chlorenchyma in green areas of a leaf, the funnel-shaped chlorenchyma cells in light areas may be more or less isodiametric (not strongly funnel shaped), with round tops toward the adaxial epidermis and variably tapering bases adjacent to the spongy cells. In these light areas, chloroplasts of these cells are not so strictly confined; indeed, some chloroplasts of these cells are present in the upper part of the cell (Fig. 4A, B, E, F). These features of leaf anatomy show that all the Begonia studied have the air space structural variegation mechanism.
Chloroplast structure and chloroplast fluorescence
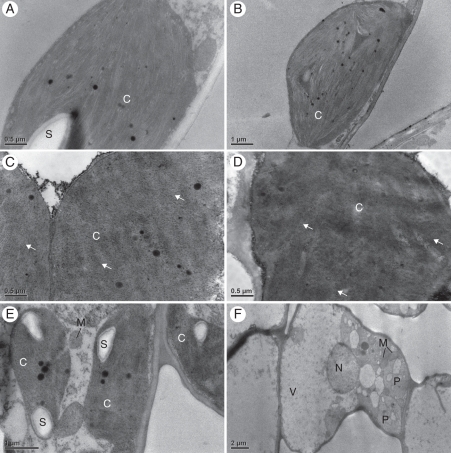
The chloroplasts from both light and green areas of the leaves of the Begonia studied did not show any observable differences. All chloroplasts had abundant thylakoid membranes, with dense stackings of grana, entirely filling the plastids (Fig. 5A–D). Starch grains appeared occasionally in sections. The maximum quantum yield of PSII, as measured by Fv/Fm, did not differ significantly between the light and green areas of a leaf (Fig. 6).
Fig. 5.
Ultrastructure of chloroplasts in the funnel-shaped chlorenchyma cells of variegated leaves of Begonia and Ficus pumila ‘Sonny.’ The left and right columns show images from green and light areas, respectively. In Begonia (A, B, B. formosana; C, D, B. pustulata), the ultrastructure of the chloroplasts in green and light areas is similar, with both areas showing abundant thylakoid membranes and dense stackings of grana (arrows). In Ficus (E, F), normal and functional chloroplasts appeared only in the green area (E) not in the light area (F). Abbreviations: C, chloroplast; M, mitochondrion; N, nucleus; P, plastid; S, starch grain; V, vacuole.
Fig. 6.
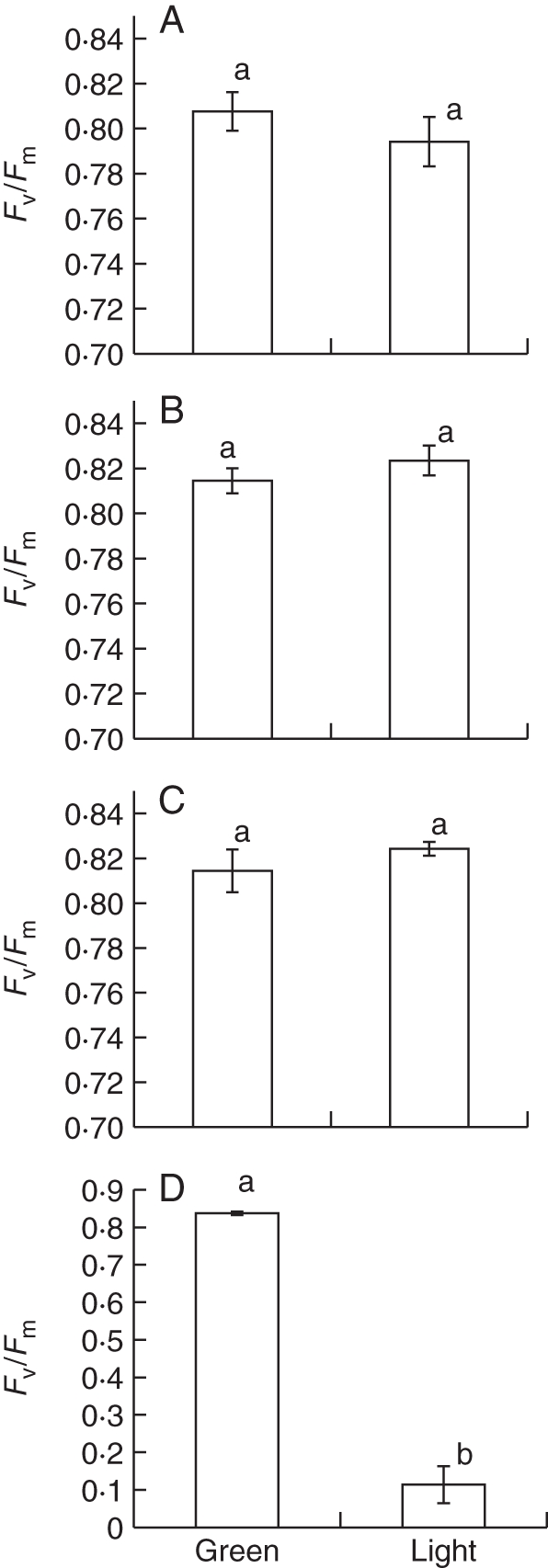
Comparisons of chlorophyll fluorescence of Fv/Fm parameters (mean ± s.e., n = 5) measured from the green and light areas of variegated leaves of Begonia and Ficus pumila ‘Sonny’. (A) Begonia formosana, (B) Begonia diadema, (C) Begonia pustulata, and (D) Ficus pumila ‘Sonny’. The maximum quantum yield of PSII in green and light areas of a variegated leaf in Begonia is similar and not significantly different (P > 0·05 in each case), but that of Ficus differs significantly (P < 0·001).
Comparison with F. pumila ‘Sonny’
Examination of the Ficus cultivar reveals in most cases very strongly contrasting effects compared with Begonia. First, the contrast between light and green areas of a leaf normally observed with reflected light is maintained under transmitted light in this Ficus (Fig. 2M–O). Secondly, the difference between the green and light areas of a Ficus leaf is due to the absence of chloroplasts in the chlorenchyma of the light areas (Fig. 4G, H). Only the chlorenchyma of the green areas has chloroplasts. In addition, the top layer of chlorenchyma has more elongated cells in the light areas than in the green areas. Moreover, in light areas of a Ficus leaf, only small plastids lacking pigment were found (Fig. 5E, F). The final feature contrasting with Begonia is that the Fv/Fm of the light and green areas differed strongly in this Ficus (P < 0·001; Fig. 6D). The only examined feature that this Ficus shares with the studied Begonia is the presence of smaller adaxial epidermal cells in light areas compared with green areas (Fig. 3G–I), but not all Begonia showed this difference.
DISCUSSION
The structural variegation that we found in the seven Begonia taxa studied has the air space type mechanism. This is the same mechanism that causes variegation in leaves of Aglaonema nitidum Kunth (Fooshee and Henny, 1990) and B. rex (Zhang et al., 2009). This mechanism involves intercellular spaces between the adaxial epidermis (or water storage tissue) and the chlorenchyma. This mechanism is associated with no evident reduction in chloroplasts or photosynthetic functioning. With this mechanism, variegation is only visible with light reflected from the adaxial surface of the leaf. In contrast, the pigment-related mechanisms of variegation, due to chlorophyll deficiency or the presence of specific pigments in the leaf tissues, remain evident under transmitted light. Examples are F. pimula ‘Sonny’ in this study, with chlorophyll deficiency, and many ornamental plants with specific pigments, such as Coleus blumei (C.-R. Sheue, pers. obs.). It is evident that an easy way to distinguish the structural mechanisms from the pigment-related variegation mechanisms is to observe the leaf from both sides with the naked eye under reflected light or with a stereoscope under transmitted light.
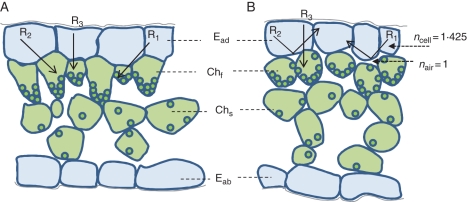
The air space type of structural variegation, studied here, can be understood from changes in the refractive index between plant cells and the intercellular space (Fig. 7). The refractive index inside a cell (ncell = 1·425) (Gausman et al., 1974) is higher than that of the intercellular space (nair = 1). The interface of the adaxial epidermis (or water storage tissue) and the intercellular space will reflect light. As Fig. 7 shows, when incident light obliquely encounters a cell–air interface over a critical angle from the normal [Snell's law, arcsin (nair/ncell) = 44·6°, R1 and R2 in Fig. 7B], it will be completely reflected (‘total internal reflection’). Lesser angles give partial reflection. Because a lot of incident light is reflected, these interfaces cause light areas on the leaf surface. This light coloration is a form of physical colour. In contrast, the absence of an air–cell interface (Fig. 7A) means no internal reflection occurs, and areas without such interfaces are a normal green colour.
Fig. 7.
Diagrammatic explanation of structural variegation due to intercellular spaces above the chlorenchyma. A change in refractive index occurs at the interface of air spaces and plant cells due to the intercellular spaces above the funnel-shaped chlorenchyma. In the green area (A), incident light at various angles is transmitted between epidermal cells and adjacent chlorenchyma without reflection at the interface. In contrast (B), when the incident light travels obliquely at angles >44·6° to the normal at the interface between a plant cell and an air space, it is completely reflected at the boundary of the cell wall (R1 and R2). Thus, the leaf colour of this area appears lighter. Note also that the funnel-shaped chlorenchyma cells in light areas (B) are more or less isodiametric, with chloroplasts not so strictly confined to the tapering base of the cell as in green areas (A). Abbreviations: Eab, abaxial epidermis; Ead, adaxial epidermis; Chf, funnel-shaped chlorenchyma; Chs, spongy chlorenchyma.
Structural variegation due to air spaces was first reported in Begonia by Hara (1957) for B. × argenteo-guttata and B. rex (the origin of many of today's ornamental variegated Begonia). Based on our present study, it seems likely that this form of variegation is general to naturally occurring Begonia. Hara (1957) proposed that light areas on the leaf surface can only be recognized when air spaces are extremely abundant. In the present study, all species except B. chlorosticta (discussed below) have clearly evident variegation with air spaces below most epidermal cells in the light areas of the leaf. Study of species such as B. nigritarum Steud., with less evident contrast between light and green areas, might throw further light on the relationship between air space abundance and the intensity of the variegation.
The attractive variegated leaf of B. chlorosticta, with large light green spots on a dark green adaxial surface, at first sight suggests yet another mechanism of variegation. The dark green parts are leaf areas without air spaces, and have normal green chlorenchyma underlaid by red-pigmented abaxial epidermis. The light green spots differ only in the presence of intercellular space between the epidermis and chlorenchyma. It is unclear why these areas are not white, as in other studied Begonia, but an answer may lie in the presence of both red pigment and chlorophyll in these areas. As red combined with green appears dark green, dark green below the air space may appear light green, rather than white. We tested this hypothesis by applying red nail polish to the abaxial epidermis of B. formosana, and found that the white areas became light green, and the green areas became dark green, in accordance with our hypothesis. However, it is also clear from the leaf structure images that B. chlorosticta has a much reduced density of air spaces in light areas compared with other variegated Begonia in this study (Supplementary Data Fig. S1), providing an alternative explanation. It may be that both phenomena contribute to the observed intensity of green in the light areas.
Structural variegation has typically been reported as resulting from intercellular space between the adaxial epidermis and the chlorenchyma (Hara, 1957; Fooshee and Henny, 1990; Tsukaya et al., 2004; Zhang et al., 2009), but in a cultivar of Begonia, we report here for the first time a variation in structural variegation where the intercellular space is located between water storage tissue and the top layer of the chlorenchyma. Both epidermis and water storage tissue are dermal tissue derived from the L1 layer of the leaf primordia (Evert, 2006). However, the chlorenchyma are derived from the L2 layer (Evert, 2006). Thus, the air space mechanism may have a natural origin in development where the L1 and L2 layers become loosely connected. In a study of tomato silvering, Grimbly (1977) suggests a possible mechanism where this loose connection between the L1 and L2 layers results from a slower cell division in the L2 layer.
Hara (1957) reported another form of structural variegation, ‘epidermis type’, due to bigger adaxial epidermal cells in light areas of a leaf. In the majority of the Begonia studied here, no appreciable cell size difference was evident between light and green areas. However, in two species (B. formosana and B. diadema), adaxial epidermal cells were smaller in the light areas (the opposite to Hara's epidermis type of variegation). The dorsal surface of the adaxial epidermis of B. chlorosticta showed a concave lens-like shape, which is a feature of deep shade plants. This feature has been seen in other species of Begonia, such as B. pavonina Ridl., Begonia ‘Kinbrook’ and B. thaipingensis King (Sheue et al., 2003). In addition, none of the Begonia studied has the typical palisade cells of most dicot leaves. Instead, they have funnel-shaped chlorenchyma with chloroplasts concentrated at the base and lateral sides of these cells. Lens-like dorsal epidermis and funnel-shaped chlorenchyma are common in deep shade plants (Harberlandt, 1912).
A feature of variegation in the Begonia of the present study is the presence of a PP (Zhang et al., 2009), which consists of striking irregular white rings visible under reflected light around adaxial epidermal cells in light areas of a leaf. Green areas of the leaf have only a very weak PP, in accordance with previous findings for B. rex (Zhang et al., 2009). Notably, we found that polygonal pattern (PP) disappears under transmitted light, as expected if this effect is caused by intercellular air spaces above the collenchyma. Moreover in B. rex, Zhang et al. (2009) found that removal of these air spaces removes any difference between PP in light and green areas of the leaf.
In our study, there is no significant difference in the maximum quantum yield of PSII measured by chlorophyll fluorescence, Fv/Fm, between the light and the green areas of the same leaf of the studied Begonia. We further examined the ultrastructure of chloroplasts between the light and the green areas of the same leaf. Both parts showed well-developed grana and thylakoid membranes entirely filling chloroplasts. The structural variegation in Begonia is able to maintain potential photosynthetic performance in light parts. In a previous study of structural variegation (Fooshee and Henny, 1990), variegated and non-variegated leaves of Aglaonema nitidum showed no differences in chlorophyll levels between light and green areas of leaves. However, Zhang et al. (2009) found that the normal green areas of B. rex leaves have higher concentrations of chlorophyll (5·30 × 10−3 mg cm−3) than the light areas (3·18 × 10−3 mg cm−3).
These findings for structural variegation in Begonia are in strong contrast to results for the chlorophyll deficiency mechanism, as confirmed here in F. pumila ‘Sonny’, in which no normal chloroplasts are found in the chlorenchyma of light areas. Almost complete loss of photosynthetic function was observed. This chlorophyll type variegation, often induced in ornamental cultivars, has a clear cost to the plant, and often has to be maintained artificially by vegetative propagation. Although this variegation mechanism can be found in natural populations, information from variegated cultivars, or variegation from other artificial means, is unlikely to be representative of natural variegation.
With the air space type of structural variegation, the reduced connections between the upper and lower layers of a leaf might be expected to reduce the strength of the leaf. However, as most leaf strength is derived from the veins, it is notable that the light areas of the variegated Begonia leaves observed in this study occur only between primary veins. In B. versicolor, no veins are light in colour. We suggest that the absence of intercellular space above primary veins maintains mechanical support in a leaf, and is another means by which the cost of variegation is limited.
It has been suggested that variegation is a defence against herbivores. Several studies have suggested that visual and shape variation in leaves can significantly reduce herbivory (Rausher, 1978; Gilbert, 1982; Mackay and Jones, 1989; Campitelli et al., 2008). Moreover, it has been suggested that egg-mimicking patterns of foliar variegation are avoided by some ovipositing insects that seek leaves free of previous eggs (Williams and Gilbert, 1981). Consistent with this herbivore defence hypothesis are observations on Hydrophyllum virginianum in natural populations where non-variegated leaves sustained nearly twice the herbivore damage of variegated leaves (Campitelli et al., 2008).
Another possible advantage of foliar variegation specifically associated with the structural mechanism is photoprotection from sunflects. Reflection from the air–cell boundary in the presence of intercellular air spaces, as described above, scatters light, possibly reducing the damaging effects of sunflects due to photoinhibition and water stress (Pearcy, 1990), and maintaining more consistent levels of photosynthesis in the leaf as a whole under varying light conditions. Esteban et al. (2008) studied Erytronium dens-canis (pigment variegation) and Pulmonaria officinalis (structural variegation) to see whether red and light green areas are more photoprotected than green areas using chlorophyll fluorescence imaging in the laboratory and the field. However, they found no effect or reduced photoprotection from high light levels in red and light green areas.
Variegated leaves are relatively common in the genus Begonia; for example, six of the 14 species native to Taiwan are variegated (B. austrotaiwanensis Chen & Peng, B. chuyunshanensis Peng & Chen, B. formosana, B. lukuana Liu & Ou, B. ravenii Peng & Chen and B. taiwaniana Hayata) (Lai, 2008). Although not all individuals may be variegated within a variegated species, it is not uncommon to find a large fraction of variegated individuals within a population. Moreover, variegated and non-variegated individuals may be found in close proximity (Supplementary Data Fig. S2; Kiew, 2005; C.-I. Peng, C.-R. Sheue and P. Chesson, pers. obs.). High frequencies of foliar variegation have also been found in other taxa, e.g. Schismatoglottis lancifolia in habitats on Gunung Gadut, Sumatra (Tsukaya et al., 2004). Although the advantages and disadvantages of variegation are not well understood, it is clear that variegated forms can persist in local populations, potentially competing with non-variegated forms of the same species. If structural variegation does indeed come with minimal costs (e.g. in photosynthesis and mechanical support), even small advantages of variegation from protection against herbivores, or from photoprotection, would be expected to lead to its evolution in the presence of suitable genetic variation. We hope that the results of this study will stimulate research into this question.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Dr Cecilia Koo of the Botanic Conservation Center (KBCC) in Pingtung, Taiwan for providing the Begonia cultivar (‘K030960’) for this study. We are grateful for discussions with Professor Maurice S. B. Ku on photosynthesis, and for comments on the manuscript from two anonymous referees. This study was partially supported by the National Science Council [NSC-97-2126-B-005-002-MY3], Taiwan, The Republic of China.
LITERATURE CITED
- Aluru MR, Bae H, Wu D, Rodermel SR. The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants. Plant Physiology. 2001;127:67–77. doi: 10.1104/pp.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campitelli BE, Stehlik I, Stinchcombe JR. Leaf variegation is associated with educed herbivore damage in Hydrophyllum virginianum. Canadian Journal of Botany. 2008;86:306–313. [Google Scholar]
- Esteban R, Fernádez-Marín B, Becerril JM, García-Plazaola JI. Photoprotective implications of leaf variegation in E. dens-canis L. and P. officinalis L. Journal of Plant Physiology. 2008;165:1255–1263. doi: 10.1016/j.jplph.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Evert RF. Esau's plant anatomy, meristems, cells, and tissues of the plant body: their structure, function, and development. 3rd edn. New Jersey: John Wiley & Sons, Inc; 2006. [Google Scholar]
- Fisher DG. Ultrastructure, plasmodesmatal frequency, and solute concentration in green areas of variegated Coleus blumei Benth. leaves. Planta. 1986;169:141–152. doi: 10.1007/BF00392308. [DOI] [PubMed] [Google Scholar]
- Fooshee WC, Henny RJ. Chlorophyll leaves and anatomy of variegated and nonvarigated areas of Aglaonema nitudum leaves. Proceedings of the Florida State Horticultural Society. 1990;103:170–172. [Google Scholar]
- Gausman HW, Allen WA, Escobar DE. Refractive index of plant cell walls. Applied Optics. 1974;13:109–111. doi: 10.1364/AO.13.000109. [DOI] [PubMed] [Google Scholar]
- Gilbert LE. The coevolution of a butterfly and a vine. Scientific American. 1982;247:110–121. [Google Scholar]
- Grimbly PE. Tomato silvering, its anatomy and chimerical structure. Journal of Horticultural Science. 1977;52:469–473. [Google Scholar]
- Haberlandt G. Physiological plant anatomy. London: MacMillan; 1912. [Google Scholar]
- Hara N. Study of the variegated leaves with special reference to those caused by air spaces. Japanese Journal of Botany. 1957;16:86–101. [Google Scholar]
- Jiang W, Zhuang M, Han H, Dai M, Hua G. Progress on color emerging mechanism and photosynthetic characteristics of colored-leaf plants. Acta Horticulturae Sinica. 2004;32:352–358. [Google Scholar]
- Kiew R. Begonias of Peninsular Malaysia. Kota Kinabalu, Malaysia: National History Publications (Borneo) Sdn. Bhd; 2005. [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence as a tool in plant physiology: II. Interpretation of fluorescence signals. Photosynthetic Research. 1984;5:139–157. doi: 10.1007/BF00028527. [DOI] [PubMed] [Google Scholar]
- Lai KS. Taiwan's native begonias and environmental needs. Nature Conservation Quarterly. 2008;63:24–30. (In Chinese) [Google Scholar]
- Mackay DA, Jones RE. Leaf shape and the host-finding behavior of two ovipositing monopfagous butterfly species. Ecological Entomology. 1989;14:423–431. [Google Scholar]
- Pearcy RW. Sunflecks and photosynthesis in plant canopies. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:421–453. [Google Scholar]
- Rausher MD. Search image for leaf shape in a butterfly. Science. 1978;200:1071–1073. doi: 10.1126/science.200.4345.1071. [DOI] [PubMed] [Google Scholar]
- Sheue CR, Saraffis V, Liu HY, Yang YP, Kiew R. Proceedings of the 24th R. O. C. Symposium on Microscopy. Taipei, Taiwan: National Defense University; 2003. Structural study on leaves of super shade plants; pp. 13–14. (Abstract) [Google Scholar]
- Spurr AR. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Okada H, Mohamed M. A novel feature of structural variegation in leaves of the tropical plant Schismatoglottis calyptrate. Journal of Plant Research. 2004;117:477–480. doi: 10.1007/s10265-004-0179-x. [DOI] [PubMed] [Google Scholar]
- de Wilde JJFE. Begoniaceae. In: Kubitzki K, editor. The families and genera of vascular plants. II. Berlin: Springer; 2011. pp. 56–71. [Google Scholar]
- Williams KS, Gilbert LE. Insects as selective agents on plant vegetative morphology: egg mimicry reduces egg laying by butterflies. Science. 1981;212:467–469. doi: 10.1126/science.212.4493.467. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hayashi T, Hosokawa M, Yazawa S, Li Y. Metallic lustre and the optical mechanism generated from the leaf surface of Begonia rex Putz. Scientia Horticulturae. 2009;121:213–217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.