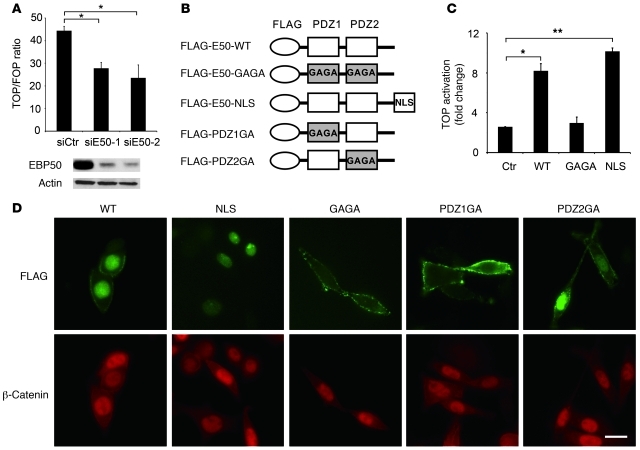
Figure 2. Nuclear EBP50 modulates Wnt/β-catenin signaling.
(A) SW480 cells were independently transfected with 40 μM siE50-1 or siE50-2, two siRNAs targeted at discrete regions of EBP50 cDNA, for 16 hours. Then, cells were trypsinized and replated into 12-well plates for 8 hours before being transfected with Top/Fop-flash reporter plasmids plus 40 μM of each EBP50 siRNA. After an additional 24 hours, cells were processed for luciferase activity assays. Western blotting demonstrated that both siRNAs efficiently suppressed the expression of EBP50. (B) The molecular configuration of the plasmids used in C and D. (C) HEK293 cells were transfected with the indicated plasmids, using empty vector pcDNA3 as a control (Ctr), and 1.5 μg of Top/Fop-flash reporter plasmid. Luciferase activity was assessed 24 hours later in triplicate. The data shown in A and C are mean ± SEM of 3 independent assays, each performed in triplicate. *P < 0.05, **P < 0.001. (D) EBP50-knockdown SW480 cells were transfected with the indicated FLAG-tagged EBP50 plasmids for 6 hours and stained with rabbit anti-FLAG (green) and mouse anti–β-catenin (red) antibodies. Scale bars: 10 μm.