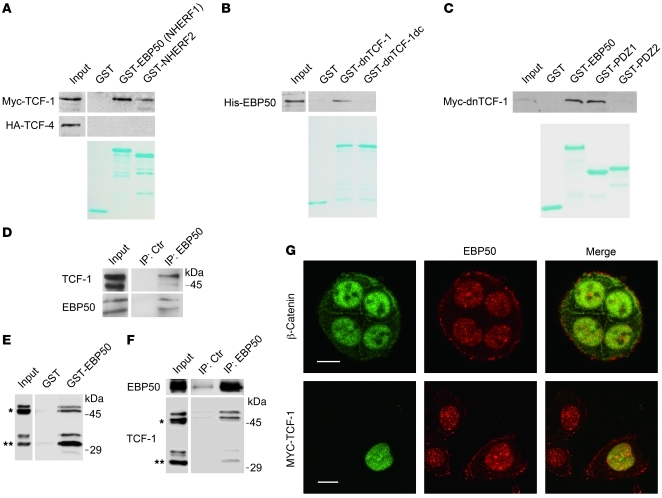
Figure 4. EBP50 interacts with TCF-1 and β-catenin through its first and second PDZ domains, respectively.
(A) Cell lysates from HEK293 cells overexpressing Myc–TCF-1 or HA–TCF-4 were incubated with glutathione beads loaded with GST, GST-EBP50 (NHERF1), or GST-NHERF2. After washing, bound materials were analyzed by immunoblotting. (B) In vitro GST pull-down assays employing purified His-EBP50 and GST, GST–dnTCF-1, or GST–dnTCF-1dc, a PDZ motif deletion mutant. (C) GST-fused full-length EBP50 (GST-EBP50) and the first (PDZ1) and second PDZ domains (PDZ2) of EBP50 were mixed with equal amounts of lysates from MDCK cells stably expressing Myc–dnTCF-1. After washing, bound materials were examined by immunoblotting. Lower panels of A–C: Coomassie blue–stained gels demonstrated the amount of each purified GST fusion protein used in the assays. (D) NCI-H28 lysates were immunoprecipitated by either control or anti-EBP50 antibody, followed by immunoblotting using TCF-1 or EBP50 antibodies. (E) Cellular lysates from Colo205 cells, which expressed both full-length (*) and N-terminally truncated TCF-1 (**), were incubated with GST or GST-EBP50 beads, and the bound material was analyzed by immunoblotting using anti–TCF-1 antibody. (F) Colo205 cell lysates were incubated with mouse anti-EBP50 or control antibody conjugated on NHS-activated beads and then processed for immunoblotting using rabbit anti-EBP50 and TCF-1 antibodies. (G) Top row: Double immunofluorescence and confocal microscopy revealed the localization of endogenous β-catenin (green) and EBP50 (red) in SW480 cells. Bottom: SW480 cells were transfected with Myc–TCF-1. Anti-Myc staining showed the colocalization of TCF-1 with endogenous EBP50 in the nucleus. Scale bars: 10 μm.