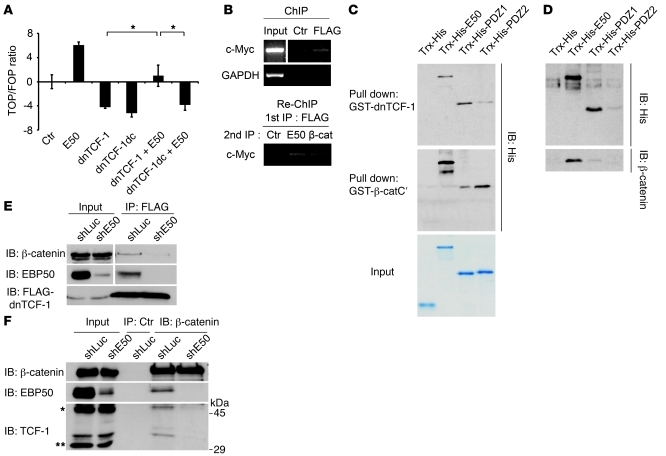
Figure 5. EBP50 promotes interaction of β-catenin and dnTCF-1.
(A) SW480 cells were transfected with 2 μg of the indicated plasmids and 0.4 μg of Top/Fop-flash reporter plasmids before luciferase activity was assessed as in Figure 2C. *P < 0.05. (B) SW480 cells stably expressing FLAG–dnTCF-1 were immunoprecipitated by either control or anti-FLAG antibody, followed by FLAG peptide elution. The eluate was then processed for Re-ChIP assay using EBP50 and β-catenin antibodies, respectively, and using rabbit anti-podocalyxin antibody as a control. Input represented 10% of total chromatin used for each experiment. (C) Purified Trx-His–fused full-length EBP50 (E50) and the first (PDZ1) and second PDZ domains (PDZ2) of EBP50 were incubated with glutathione beads conjugated with GST–dnTCF-1 (top panel) and GST–β-catenin C-terminal (middle panel) recombinant proteins. After incubation and washing, bound materials were assessed by immunoblotting. The amount and purity of the recombinant proteins are shown by a Coomassie blue–stained gel in the bottom panel. (D) Glutathione beads conjugated with GST–dnTCF-1 were incubated with the indicated Trx-His fusion proteins for 1 hour. Then untagged β-catenin, which was released from the GST–β-catenin by thrombin digestion, was added in the mixture for 1 more hour before the pulled-down materials were examined by immunoblotting. (E) SW480 cells stably expressing FLAG–dnTCF-1 were infected with lentivirus expressing siRNA against EBP50 (shE50) or luciferase control (shLuc). The cellular lysates were immunoprecipitated by mouse anti-FLAG antibody and processed for immunoblotting using the indicated antibodies. (F) Colo205 cells were infected with lentivirus expressing siRNA against EBP50 or luciferase control. The cellular lysates were immunoprecipitated by rabbit anti–β-catenin antibody and processed for immunoblotting using mouse anti–β-catenin, anti-EBP50, and anti–TCF-1 antibodies.