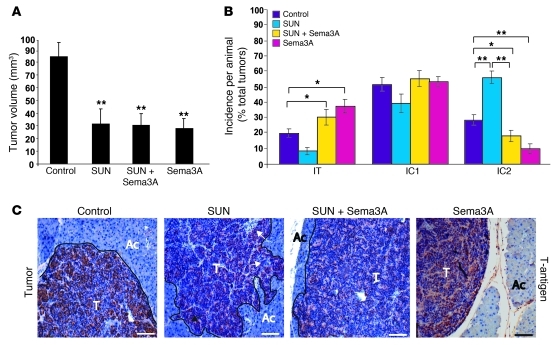
Figure 1. Sema3A blocks tumor invasion caused by antiangiogenic treatment.
(A) Total tumor volume in 4-week treatment regression trial (from 12 to 16 weeks of age) showed that sunitinib (SUN), AAV8-Sema3A in combination with sunitinib, and AAV8-Sema3A reduced tumor burden 64%, 63%, and 66%, respectively, compared with controls (AAV8-LacZ–injected RIP-Tag2 treated with vehicle; see Methods). (B) Percent encapsulated (IT), microinvasive (IC1), and fully invasive (IC2) carcinomas. Combined AAV8-Sema3A and sunitinib decreased IC2 carcinoma incidence 62% compared with sunitinib alone. Sema3A-treated tumors showed a statistically significant 64% decrease of IC2 carcinomas compared with controls. (C) Analysis of tumor invasiveness by means of SV40 T-antigen immunostaining. Sunitinib-treated tumors displayed an invasive front extensively intercalated into the surrounding tissue (arrows). T, tumor; Ac, acinar tissue. *P < 0.05, **P < 0.01, unpaired Mann-Whitney U test. Scale bars: 50 μm.