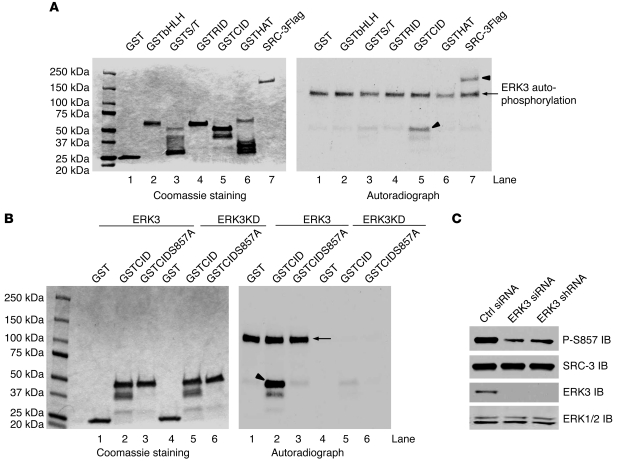
Figure 2. ERK3 phosphorylates SRC-3 at S857.
(A) ERK3 phosphorylates SRC-3 at the CID region in vitro. SRC-3Flag protein was expressed in Sf9 cells and purified using anti-Flag beads. The N-terminal basic helix-loop-helix domain-containing region of SRC-3 (aa 1–320), serine/threonine-rich region (S/T; aa 321–580), receptor interaction domain-containing region (aa 581–840; lane 5), CID region (aa 841–1080), and histone acetyltransferase domain-containing region (HAT; aa 1081–1424) were expressed as GST-fusion proteins in E. coli. In vitro kinase assay was performed by incubating purified SRC-3Flag (lane 7) or GSTSRC-3 fragment proteins (see Coomassie staining) with purified ERK3 kinase. ERK3 autophosphorylation (arrow, lane 7) and phosphorylation of the substrates (arrowheads, lane 5 and 7) are shown in the autoradiograph in the right panel. (B) ERK3 phosphorylates SRC-3 at S857 within the CID region in vitro. GST, GSTCID, and GSTCIDS857A proteins (see Coomassie staining) were incubated with either purified active ERK3 protein or ERK3KD protein. The arrow and arrowhead indicate ERK3 autophosphorylation and GSTCID phosphorylation, respectively. (C) Knockdown of ERK3 decreases SRC-3 phosphorylation at S857 in H1299 cells. ERK3 was knocked down by transfecting cells with ERK3 siRNA or by transducing cells with lentiviruses expressing ERK3 shRNA. SRC-3 phosphorylation at S857 was analyzed by Western blotting using an Ab that specifically recognizes phosphorylated S857.