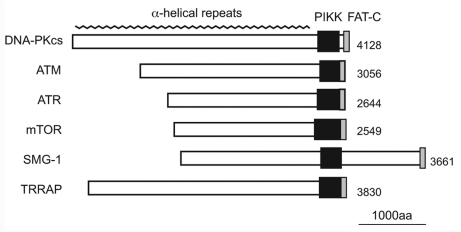
Figure 1. The domain structures of human PIKK family members. PIKKs share the highly conserved catalytic PIKK domain and the FAT-C (FRAP, ATM, and TRRAP C-terminal) domain. Although the PIKK domain has sequence homology to the catalytic domain of PI3-kinases, PIKKs act as Ser/Thr protein kinases except for TRRAP. The FAT-C domain located near the PIKK domain is thought to modulate the kinase activity. The N-terminal region of PIKK is composed of α-helical repeats, which contribute to protein-protein interactions.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.