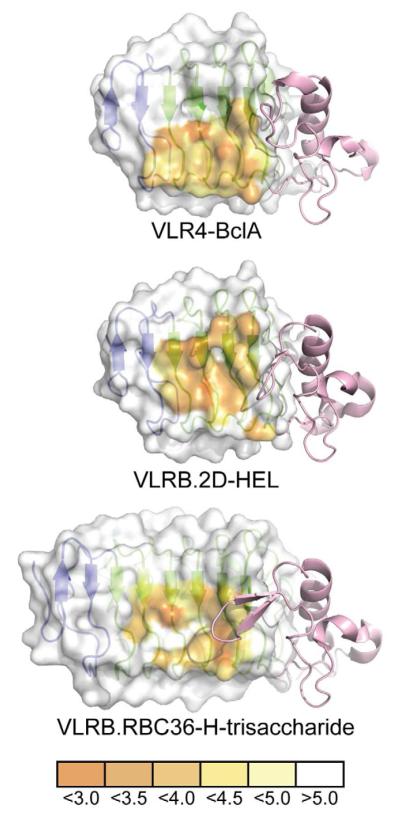
Figure 5. Comparison of VLRB Binding Interfaces with Different Antigens.
The concave surface of each VLRB is represented as a surface highlighting the proximity of each amino acid to its respective antigen in angstroms. The surface used by VLRB to contact antigens favors the use of the C-terminal LRR motifs and VLR4 is heavily biased towards the used of the C-terminal ends of the ß-strands of these motifs. The LRRCT motif (pink) of each VLRB shows dramatically different lengths and conformations in their LRRCT-loops.