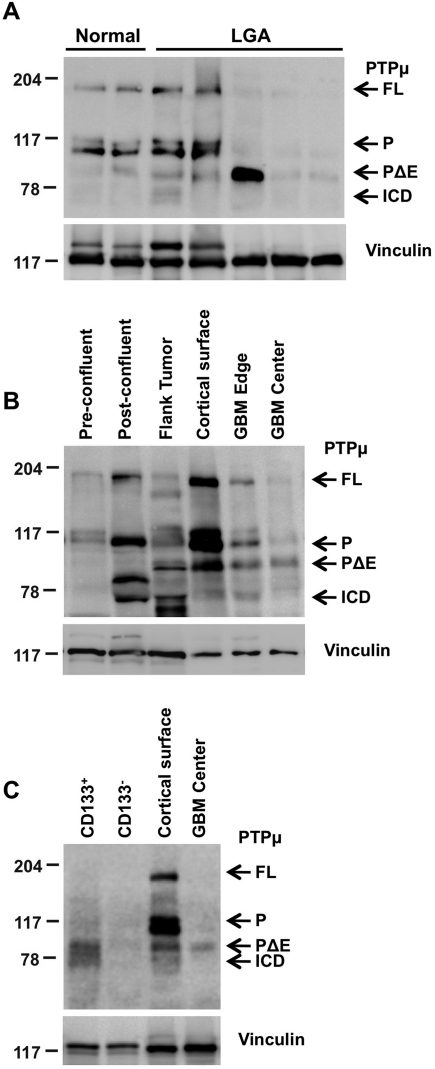
Fig. 1.
PTPµ proteolysis varies with astrocytoma grade, growth conditions, and in glioma stem-like cells. Lysates were separated by 8% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes and immunoblotted using antibodies against the intracellular segment of PTPµ (SK18). Immunoblots were stripped and reprobed for vinculin to control for loading. Full-length PTPµ (FL) is detected at 200 kDa on an immunoblot. Full-length PTPµ is normally cleaved into 2 ∼100-kDa fragments that remain noncovalently associated. The intracellular segment (P) is detected at ∼100 kDa and is further proteolytically cleaved into PΔE and ICD fragments (∼83 and 74 kDa, respectively) in gliomas. (A) Relative expression of full-length PTPµ and PTPµ intracellular fragments from noncancerous brain tissue (epilepsy), a cortical surface sample from noncancerous brain tissue of a GBM patient, and LGAs are shown. (B) Expression of full-length PTPµ and PTPµ fragments in parental U-87 MG cells growing either in vitro in 2D tissue culture dishes at pre- and postconfluent states or in vivo in flank tumor xenografts. Matched normal cortical surface sample from nontumor brain tissue of a GBM patient (cortical surface) and cancerous tissue from the edge and center of a tumor (GBM edge and center) are shown as controls for the expression of full-length PTPµ and PTPµ fragments. (C) Expression of PTPµ fragments in CD133+ cells compared with CD133− cells.