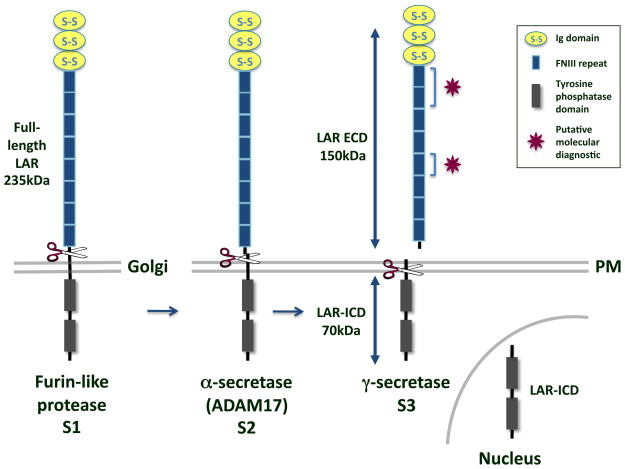
Figure 1.
LAR cleavage. The type I transmembrane protein LAR RPTP is composed of 3 Ig domains and 8 FNIII repeats in its ECD and two tandem tyrosine phosphatase domains in its ICD. It is cleaved by a furin-like endoprotease in the Golgi to yield a 150kDa E-subunit and an 85kDa P-subunit that remain non-covalently attached (S1) [36, 37]. In response to phorbol esters, high cell density, or calcium ionophores, a 150kDa segment of the LAR ECD is cleaved at the plasma membrane (PM) and shed by ADAM-17/TACE (S2) [40]. The γ-secretase complex then cleaves the transmembrane domain of LAR to yield a 70kDa membrane-free fragment, LAR-ICD, which is capable of translocating to the nucleus (S3) [31]. Molecular diagnostics like the ones used to identify extracellular PTPμ fragments in GBM tissue could be designed to bind to the heterophilic [4, 6] and homophilic [9] binding sites on LAR, as indicated by the magenta stars.