Abstract
AIM: To study the effects of probiotic metabolites on maturation stage of antigen-presenting immune cells.
METHODS: Ganeden Bacillus coagulans 30 (GBC30) bacterial cultures in log phase were used to isolate the secreted metabolite (MET) fraction. A second fraction was made to generate a crude cell-wall-enriched fraction, by centrifugation and lysis, followed by washing. A preparation of MET was subjected to size exclusion centrifugation, generating three fractions: < 3 kDa, 3-30 kDa, and 30-200 kDa and activities were tested in comparison to crude MET and cell wall in primary cultures of human peripheral blood mononuclear cell (PBMC) as a source of antigen-presenting mononuclear phagocytes. The maturation status of mononuclear phagocytes was evaluated by staining with monoclonal antibodies towards CD14, CD16, CD80 and CD86 and analyzed by flow cytometry.
RESULTS: Treatment of PBMC with MET supported maturation of mononuclear phagocytes toward both macrophage and dendritic cell phenotypes. The biological activity unique to the metabolites included a reduction of CD14+ CD16+ pro-inflammatory cells, and this property was associated with the high molecular weight metabolite fraction. Changes were also seen for the dendritic cell maturation markers CD80 and CD86. On CD14dim cells, an increase in both CD80 and CD86 expression was seen, in contrast to a selective increase in CD86 expression on CD14bright cells. The co-expression of CD80 and CD86 indicates effective antigen presentation to T cells and support of T helper cell differentiation. The selective expression of CD86 in the absence of CD80 points to a role in generating T regulatory cells.
CONCLUSION: The data show that a primary mechanism of action of GBC30 metabolites involves support of more mature phenotypes of antigen-presenting cells, important for immunological decision-making.
Keywords: Mononuclear phagocytes, Dendritic cell maturation, Co-stimulatory molecules, Antigen-presentation, Probiotics, Metabolites
INTRODUCTION
Bacteria are ubiquitous in the environment, having colonized every extreme of nature. This includes the human body where they outnumber human cells by an order of magnitude. The biggest reservoir of these symbiotic bacteria on the human body is the lower gastrointestinal tract[1] where large numbers of coexisting (commensal) bacteria participate in nutrient assimilation including the breakdown of indigestible carbohydrates. They also produce amino acids and vitamins for their host and play a key role in healthy immune system development.
The immune system recognizes both pathogenic and commensal bacteria through a family of pattern recognition receptors known as the toll-like receptor (TLR) family[2]. These receptors interact with molecules present on the exterior surface of bacteria and include lipopolysaccharide (LPS), flagellin, lipoteichoic acid and lipoproteins as well as bacterial DNA. Toll-like receptors are present on cells participating in both innate and adaptive immunity such as monocytes/macrophages and dendritic cells (DC) as well as epithelial cells of the intestinal mucosa.
The emerging picture is that commensal bacteria have an enormous impact on health. While a healthy microbiota can aid the host by increasing nutrient absorption and training the immune system to not respond to self, conversely an unhealthy (i.e., unbalanced) microbiota can lead to malabsorption, inflammation and disease[3-5]. A growing body of evidence suggests that these effects, both positive and negative, of the microbiota on the host are mediated by the immune system. Probiotics are defined as microorganisms that when ingested in a sufficient amount confer a health benefit upon the host and are known to interact with the immune system. Probiotic microorganisms have a long history of human consumption in the form of fermented foods and have shown health benefits in treating dysbiosis, irritable bowel syndrome, and eczema[6]. GanedenBC30™(Bacillus coagulans GBI-30, 6086) (GBC30) is a proprietary strain of the gram positive, lactic acid producing spore-forming bacteria known as Bacillus coagulans. This strain of B. coagulans can survive extremes of heat and pressure in manufacturing as well as the harsh, acidic environment of the human gastrointestinal tract, leading to a very high survival rate and germination in the lower intestinal tract. The safety of consumption of this strain was documented in acute and sub-chronic studies in rats[7].
One way in which commensal bacteria modulate the immune response is by the secretion of certain bioactive compounds. This suggests that metabolites of commensal bacteria have effects of their own and that there may be unique health benefits to be derived from the consumption of live probiotic cultures or probiotic metabolite preparations. Recent studies on the bacterial compound polysaccharide A from Bacteroides fragilis have shown the ability of this molecule to prevent intestinal inflammation caused by Helicobacter pylori infection and to correct the symptoms of encephalomyelitis in mice, an animal model for human multiple sclerosis[8-10]. The recent sequencing data from 178 commensal microbial genomes has identified over 30 thousand potential protein-coding sequences of which 97% are unique[11]. This suggests a vast untapped reservoir of novel genes including those coding for potential secreted compounds.
The work presented here build on a previous study that showed both enhancement of innate immune responses as well as anti-inflammatory effects of GBC30 in vitro[12]. In particular, the data presented here has aimed at investigating the differences between a crude preparation versus the metabolite fraction in more detail with a particular focus on modulation of key regulatory immune cells by specific size-selected fractions of GBC30 metabolite (MET) compounds.
MATERIALS AND METHODS
Reagents
The following buffers and reagents were obtained from Sigma-Aldrich (St. Louis, MO): Histopaque 1077 and 1119, phosphate-buffered saline (PBS), RPMI-1640 culture medium, fetal calf serum, L-glutamine 200 mmol/L, penicillin-streptomycin 100X solution, and bovine serum albumin. CD80-FITC, CD86-PE, CD16-PE and CD14-PerCP were obtained from BD Biosciences (San Jose, CA). Sodium Azide (NaN3) was obtained from LabChem Inc. (Pittsburgh, PA). Low-binding 100 μm zirconium beads were obtained from OPS Diagnostics (Lebanon, NJ) and 0.2 μm cellulose acetate filters from Whatman (Florham Park, NJ). The Bacillus coagulans strain (GanedenBC30™) was obtained from Ganeden Biotech Inc. (Mayfield Height, OH).
Preparation of Bacillus coagulans metabolite fractions
Using sterile technique, two separate samples of 2.0 g of GanedenBC30™spores were each placed into 25 mL PBS and heated at 70 °C for 30 min. Spores were then centrifuged at 2400 rpm for 5 min, PBS was removed and each tube of spores re-suspended and placed in culture flasks containing 25 mL of RPMI-1640 culture medium. The cultures were incubated at 37 °C for 24 h at which time an additional 20 mL of RPMI-1640 was added and the cultures incubated for an additional 24 h. Following 48 h of incubation, the bacterial cultures contained 5 × 107 bacteria/mL.
Preparation of GanedenBC30™ culture supernatant as a source of metabolites (MET): Cultures were transferred to 50 mL centrifuge tubes and initially spun at 1000 rpm for 2 min to remove any remaining spores. The liquid containing the bacteria and metabolites was decanted into new tubes and centrifuged at 3500 rpm for 20 min. The supernatant was decanted from the large bacterial pellets and combined in a single tube followed by filtration twice through a 0.2 μm cellulose acetate syringe filter. This filtrate was either frozen directly as 250 μL aliquots (crude metabolite fraction) or spun through Amicon Ultra protein size separation columns from Millipore (Bedford, MA) followed by aliquot preparation and storage at -20 °C. Separation of the metabolites into different molecular weight fractions was performed in the following manner: for the fraction that is < 3 kDa, the crude metabolite preparation was placed onto a 3 kDa molecular weight cutoff centrifuge column and spun at 2500 rpm for 10 min. The filtrate that passed through the filter contained material that was less than 3 kDa. Aliquots were made from this material and frozen (metabolites < 3 kDa fraction). The material that did not pass through the column was then placed into a tube containing a 30 kDa molecular weight cutoff filter and spun at 2500 rpm for 10 min. The filtrate that passed through this filter contained material that was 3-30 kDa. Aliquots were made from this material and frozen (metabolites 3-30 kDa fraction). The remaining metabolite material that did not pass through the 30 kDa molecular weight filter was also aliquoted and frozen and this fraction was called metabolites 30-200 kDa fraction. It is important to note that these size separations are not exact and that while large molecules are excluded from the fractions containing the smaller molecules, some small molecules may still remain in the fractions containing the larger molecules.
Preparation of GanedenBC30™ crude cell wall (CW): The two bacterial pellets were each processed separately and then combined after the final bead milling step. Following centrifugation of the bacterial culture at 3500 rpm for 20 min and decanting of the supernatant, the bacterial pellets were washed twice in 45 mL of PBS with subsequent pelleting by centrifugation at 2500 rpm for 10 min. The washed pellets went through one freeze/thaw cycle followed by multiple bead milling cycles. In brief, the pellets were resuspended in 4 mL of PBS and then 4 mL of 100 μm low-binding zirconium beads were added. One cycle of bead milling consisted of 60 one-second pulses of the bacteria/bead mixture on a vortex mixer. Ten of these cycles were performed. The bacteria/bead mixture was placed on ice in between bead milling cycles. At the end of the 10 bead-milling cycles, the beads were allowed to settle in the tubes and the liquid removed from the two tubes and combined. The liquid containing the fragmented bacteria was spun at 3500 rpm for 20 min followed by transfer of the liquid to Eppendorf tubes and centrifugation at 14 000 rpm for 5 min. The high speed centrifugation was necessary to remove any large fragments of bacteria that were not disrupted by the bead milling. The final solution was brought up to 45 mL with PBS and filtered through a 0.2 μm cellulose acetate filter and stored directly at -20 °C as 250 μL aliquots (crude cell wall). It is important to note that the cell wall preparation will also contain some GBC30 cellular contents in addition to cell wall components.
Purification of peripheral blood mononuclear cells
Healthy human volunteers between the age of 20 years and 60 years served as blood donors after obtaining written informed consent, as approved by the Sky Lakes Medical Center Institutional Review Board. Isolation of peripheral blood mononuclear cell (PBMC) was performed as previously described[13].
Cell surface staining of CD14 positive mononuclear phagocytes
Complete cell culture media used for the culture of PBMC consisted of RPMI-1640 supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. Peripheral blood mononuclear cells were cultured for 3 d in the presence of serial dilutions of BC30 metabolite fractions or crude cell wall followed by cell surface immunostaining with CD14, CD80, CD86 and CD16 monoclonal antibodies. Processing of cells for immunostaining was performed as previously described[13] with the following modifications: optimal amounts of monoclonal antibodies per sample were 3 μL for CD14-PerCP and 4 μL for CD80-FITC, CD86-PE and CD16-PE. Experiments were performed three times using PBMC isolated from three different blood donors. Each test condition was performed in duplicate and untreated and LPS-treated controls were tested in quadruplicate and triplicate, respectively.
Statistical analysis
Statistical significance was tested using Student’s t-test performed with Microsoft Excel. All P values were two-sided and were considered significant when P < 0.05 and highly significant when P < 0.01. Only statistically significant P values are reported.
RESULTS
GBC30 effects on mononuclear phagocyte differentiation
Mononuclear phagocyte differentiation in 3-d primary PBMC cultures was examined by cell surface staining for proteins expressed by monocytes/macrophages and dendritic cells. These included CD14 (Figure 1) and CD80 and CD86 (Figures 2 and 3). CD14 expression on CD14bright cells was increased following exposure of cells to GBC30 crude MET and CW fractions (Figure 1A). Treatment of cells with crude MET showed a strong dose-dependent response with statistically significant increases occurring with the 4 highest doses. As expected, LPS treatment of cells greatly increased CD14 expression[14]. Crude CW also led to statistically significant increases in CD14 expression. When the effects of crude and size-selected MET fractions of GBC30 at a 1:200 dilution were compared (Figure 1B), high molecular weight fractions (crude and 30-200 kDa) increased CD14 expression while PBMC treated with either the 3-30 kDa or < 3 kDa fractions showed CD14 expression levels similar to untreated cultures.
Figure 1.
CD14 expression on mononuclear phagocytes. Mononuclear phagocytes present in 3-d peripheral blood mononuclear cell cultures exposed to either the Ganeden Bacillus coagulans 30 (GBC30) metabolites (MET), cell wall-enriched (CW), or MET fractions, were identified using electronic gating of the flow cytometry data by gating on forward scatter/side scatter followed by gating for CD14 positivity. A comparison was made between cells that were untreated (UT), exposed to lipopolysaccharide (LPS) or to the different GBC30 fractions. A: Comparison of CD14 mean fluorescence intensity showed a dose-dependent increase in CD14 expression in cells treated with crude MET. A milder increase was seen for cells treated with crude CW. The baseline indicates CD14 expression on untreated cells; B: The increase in CD14 expression was primarily caused by high molecular weight compounds present in MET; C: The percent of CD14bright cells in the mononuclear phagocyte population was decreased by all fractions of MET; D: The percent of CD14dim cells in the mononuclear phagocyte population was increased by treatment of cells with all MET fractions. Bar graphs show data from 1:200 dilutions of each MET fraction and lipopolysaccharide (1 μg/mL). aP < 0.05, bP < 0.01 and cP < 0.001. For each data point, the mean + SD are shown for each duplicate data set. Graphs show data representative of 1 out of 3 experiments. MFI: Mean fluorescence intensity.
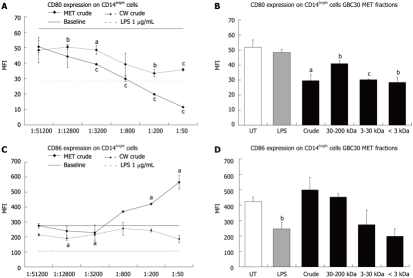
Figure 2.
Expression of the co-stimulatory molecules CD80 and CD86 on CD14bright mononuclear phagocytes from 3-d peripheral blood mononuclear cell cultures. A: Comparison between the effects of serial dilutions of Ganeden Bacillus coagulans 30 (GBC30) crude metabolites (MET) or cell wall enriched (CW) fractions on CD80 expression on CD14bright cells showed dose-dependent decreases in CD80 expression. Both MET and CW reduced CD80 expression to levels similar to those seen with Lipopolysaccharide (LPS) treatment; B: A comparison of the effects of size-fractionated MET on CD80 expression on CD14bright cells shows that all MET fractions reduce expression; C: Comparison between the effects of serial dilutions of crude MET or CW on CD86 expression on CD14bright cells showed dose-dependent increases in CD86 expression when cells were exposed to the three most concentrated dilutions of MET. Treatment of cells with CW resulted in a uniform modest decrease in CD86 expression; D: The effect on increased CD86 expression is present only in the crude preparation of MET. Bar graphs show data from 1:200 dilutions of each MET fraction and lipopolysaccharide (1 μg/mL). aP < 0.05, bP < 0.01 and cP < 0.001. For each data point, the mean + SD are shown for each duplicate data set. Graphs show data representative of 1 out of 3 experiments. MFI: Mean fluorescence intensity; UT: Untreated.
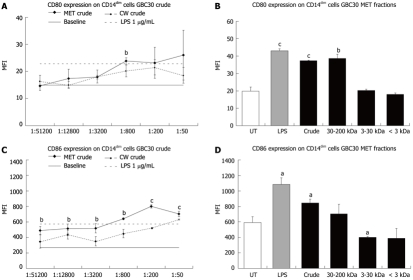
Figure 3.
Expression of the co-stimulatory molecules CD80 and CD86 on CD14dim mononuclear phagocytes from 3-d peripheral blood mononuclear cell cultures. A: Comparison between the effects of serial dilutions of Ganeden Bacillus coagulans 30 (GBC30) crude metabolites (MET) or cell wall enriched (CW) fractions on CD80 expression on CD14dim cells showed that both MET and CW led to increased expression; B: The increase in CD80 expression following treatment of cells with MET was due to high molecular weight compounds; C: Comparison of CD86 expression on CD14dim cells exposed to crude fractions of MET or CW resulted in increased CD86 expression; D: Size-selected fractions of MET did not have uniform effects on CD86 expression on CD14dim cells. Crude MET increased expression while 3-30 kDa and < 3 kDa fractions decreased expression. Bar graphs show data from 1:200 dilutions of each MET fraction and lipopolysaccharide (1 μg/mL). aP < 0.05, bP < 0.01 and cP < 0.001. For each data point, the mean + SD are shown for each duplicate data set. Graphs show data representative of 1 out of 3 experiments. MFI: Mean fluorescence intensity; UT: Untreated; LPS: Lipopolysaccharide.
Reduction of CD14bright cells
Because CD14 expression on mononuclear phagocytic cells varies and expression levels have been correlated with different cell populations, the percent of CD14bright versus CD14dim cells was determined for PBMC cultures exposed to different GBC30 MET fractions. All fractions of MET at a 1:200 dilution led to decreased numbers of CD14bright cells (Figure 1C) while an inverse pattern of response was seen regarding changes in CD14dim cell numbers (Figure 1D). In this case all fractions of MET led to increases in the percent of CD14dim cells.
CD14bright cells: Effects of GBC30 metabolite and cell wall on CD80 and CD86 expression
Next, CD80 and CD86 expression was determined for the CD14bright cell population. Both MET and CW crude fractions led to statistically significant decreases in CD80 expression on CD14bright cells (Figure 2A) while only MET crude increased CD86 expression (Figure 2C). When crude and size selected fractions of MET were compared at the 1:200 dilution all fractions of MET led to similar statistically significant decreases in CD80 expression (Figure 2B). A comparison of the effect of MET fractions on CD86 expression showed that the 3-30 kDa and < 3 kDa fractions led to a decrease but these changes were not statistically significant (Figure 2D).
CD14dim cells: Effects of GBC30 metabolite and cell wall on CD80 and CD86 expression
When expression of the co-stimulatory molecules CD80 and CD86 was determined for the CD14dim cell population, both MET and CW crude increased CD80 (Figure 3A) and CD86 (Figure 3C) expression with MET crude having the biggest effect, particularly on CD86 expression. When crude and size selected fractions of MET were compared at the 1:200 dilution, only the crude and 30-200 kDa fractions led to statistically significant increases in CD80 expression (Figure 3B). A comparison of the effect of MET fractions on CD86 expression showed that crude MET increased CD86 expression while the 3-30 kDa and < 3 kDa fractions decreased expression (Figure 3D).
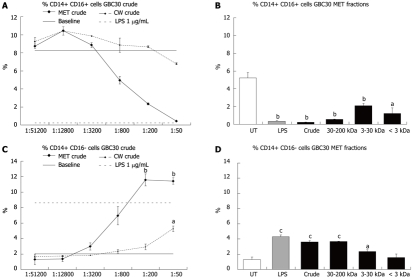
Reduction in CD14+ CD16+ cells
Mononuclear phagocytes have also been classified according to expression of the cell surface protein CD16 with CD14+ CD16+ cell subsets considered to be pro-inflammatory[15]. The effect of crude MET and CW fractions on the percent of CD14+ CD16+ and CD14+ CD16- cells in 3-d PBMC cultures was investigated. Crude MET treatment of cells resulted in a dose dependent decrease in CD14+ CD16+ cells (Figure 4A) and an increase in CD14+ CD16- cells (Figure 4C). Crude CW had a much milder effect that mirrored that of crude MET. When cells were exposed to the crude or size fractionated preparations of MET at a 1:200 dilution, it was the fractions with the largest compounds (crude and 30-200 kDa) that showed the greatest effect on CD14+ CD16+ (Figure 4B) and CD14+ CD16- (Figure 4D) cell numbers although the 3-30 kDa and < 3 kDa fractions also produced statistically significant reductions in the number of CD14+ CD16+ cells.
Figure 4.
Changes in the percent of CD14+ CD16+ and CD14+ CD16- cell populations following exposure of 3-d peripheral blood mononuclear cell cultures to Ganeden Bacillus coagulans 30. A: Exposure of cells to serial dilutions of Ganeden Bacillus coagulans 30 (GBC30) crude metabolites (MET) led to a strong dose-dependent decrease in CD14 CD16 double positive cells while exposure to cell wall enriched (CW) did not reduce this cell population; B: Treatment of cells with size-selected MET fractions show that all fractions of MET reduce the number of CD14+ CD16+ cells; C: The percent of CD14+ CD14- cells in peripheral blood mononuclear cell cultures increased in cultures treated with crude MET and CW. Treatment with MET resulted in a very strong dose-dependent increase while CW treatment produced a milder increase at the two highest concentrations; D: Treatment of cells with size-selected MET fractions show that only fractions containing high molecular weight compounds increase the number of CD14+ CD16- cells. Bar graphs show data from 1:200 dilutions of each MET fraction and lipopolysaccharide (1 μg/mL). aP < 0.05, bP < 0.01 and cP < 0.001. For each data point, the mean + SD are shown for each duplicate data set. Graphs show data representative of 1 out of 3 experiments. UT: Untreated; LPS: Lipopolysaccharide.
DISCUSSION
The work presented here investigated the effects of MET and CW fractions of the GBC30 probiotic strain on mononuclear phagocyte phenotypes in primary PBMC cultures. The cellular model for examining the immune effects was carefully chosen, and primary PBMC cultures were used because this allows the simultaneous interaction of multiple cell types and has been shown to support the survival of blood dendritic cells without the addition of exogenous cytokines[16]. One of the main findings was the biological activities of the metabolites and the data showed that a primary mechanism of action of BC30 metabolites involved support of more mature phenotypes of antigen-presenting cells, important for immunological decision-making.
Compounds present in the MET crude fraction consisted entirely of compounds that were secreted by GBC30 into the culture media. The CW crude fraction was isolated from whole bacteria and may contain some compounds present in the MET preparation in addition to compounds unique to the cell wall. Size fractionation of crude MET was used to evaluate immune modulating compounds based on MW and their association with one or more fractions.
Probiotic organisms support mucosal immunity and similar to commensal bacteria in the human gut, they interact with mononuclear phagocytic cells such as dendritic cells and macrophages[17-19]. The expression levels of CD80 and CD86 co-stimulatory molecules can be used to indicate the differentiation of mononuclear phagocytes to that of antigen presenting cells such as dendritic cells. While CD14 is still present on some subsets of dendritic cells, typically when mononuclear phagocytes adopt a dendritic cell identity, CD14 expression is down regulated with the concurrent up regulation of CD80 and CD86[20]. The differential roles of the co-stimulatory molecules CD80 and CD86 suggests that co-expression of both molecules on dendritic cells leads to T helper cell differentiation, whereas the predominant expression of CD86 support T regulatory cells, and supports an anti-inflammatory cytokine profile by decreasing Interferon-gamma production and increasing interleukin (IL)-4 production[21]. Since the current literature suggests that mononuclear phagocytes present in the circulation are already committed in their developmental path[22], the changes seen in CD14 expression suggest that MET and CW simultaneously enhance the maturation of two separate subpopulations of mononuclear phagocytic cells (CD14bright and CD14dim) towards their corresponding macrophage and dendritic cell phenotypes. The effect of GBC30 on putative DC maturation in PBMC cultures, suggests that DC may be responsible for the IL-6 production that was previously shown in vitro[12], and this increased IL-6 production may reflect normal physiological interactions between DC and commensal bacteria in the human gut[17,23].
The data suggest that live GBC30 in the gut lumen would provide metabolites from GBC30, different from the immune modulating compounds associated with the cell wall enriched fraction, and support the interpretation that the live metabolically active GBC30 has stronger immune modulating activity than accounted for by its cell wall alone. Immune modulating activity has been identified from the supernatant of the probiotic strains Lactobacillus casei Shirota[24] and Bifidobacterium breve[25], the probiotic yeast Saccharomyces boulardii[26], the commensal bacterium Faecalibacterium prausnitzii[27] and gut-derived lactobacilli and bifidobacteria[28]. In the case of Faecalibacterium prausnitzii, injection of the supernatant completely protected mice from trinitrobenzenesulphonic acid induced colitis while live bacteria provided only partial protection[27]. Most of these studies focused on cytokine production in monocyte-derived dendritic cell cultures[26,27] and have determined this to occur through a TLR2 dependent mechanism. In one study, it was determined that the active component in the supernatant from Lactobacillus casei was a polysaccharide peptidoglycan complex[24] while another study has suggested that the immune boosting effect of common botanical extracts is through effects of bacterial lipoproteins and lipopolysaccharides (derived from endophytes, the resident bacteria present in all plants) on macrophage activation[29].
Thus, due to direct effects on mononuclear phagocyte differentiation, GBC30 metabolites lend support to two important cell types responsible for antigen recognition, presentation to cells within the adaptive immune system, and execution of regulatory functions, including immunological memory. The effect of dried/reconstituted material was tested in three different bioassays previously reported to show bioactivity[12], including anti-inflammatory effects (data not shown), and no significant difference was seen between this and frozen/thawed material. The stability of the bioactive compounds in the metabolite fraction holds promise for development of a consumable product.
Results from the GBC30 MET fractions suggest that the metabolic activity of this probiotic organism is an intregral part of its immune modulating functions, and that multiple different compounds act in synergy to support key aspects of mucosal immune protection. These results suggest specific mechanisms of action and may give insight into some aspects of previous clinical studies showing reduced symptoms from irritable bowel syndrome[30]. We suggest that further studies include ex vivo evaluation of mononuclear cells isolated from lamina propria and Peyer’s patches, in terms of antigen presentation, dendritic cell and B lymphocyte maturation, and IgA production. Further clinical work is warranted, not only in populations with inflammatory syndromes, but also in populations with reduced mucosal immune protection, and should include assessment of inflammatory markers in serum, as well as secretion of IgA.
In conclusion, the biological activities reported here for the metabolites point to a unified mechanism of action directed at the differentiation and maturation state of antigen-presenting cells such as the macrophage/dendritic cells. In terms of immune regulation, this plays a pivotal role in decision-making, for example in whether T lymphocytes are induced into immunological anergy (unresponsiveness, tolerance) or whether they are triggered into proliferation, cytokine production, and other mechanism of inter-cellular communication. It is conceivable that metabolites are absorbed into the mucosal immune tissue along the intestinal track and help direct more efficient antigen-recognition, while reducing immune reactivity towards harmless food-borne antigens. This may provide a mechanism to explain the improved immune protection, while also seeing a reduction in food allergies and associated inflammatory reactions with consumption of certain probiotic strains.
COMMENTS
Background
The mucosal surface of the human gastrointestinal tract is an interface between the external and internal environments, separated by a single epithelial cell layer. On the one side are food antigens, commensal bacteria and potential pathogens while cells of the immune system reside on the other. Oral tolerance refers to the ability of the immune system to not react towards food and commensal bacterial antigens while still evoking a robust immune response towards pathogens. Probiotic bacteria interact with the host immune system and elicit beneficial immune modulating effects that include a reduction in inflammation in inflammatory bowel disease, amelioration of antibiotic-induced diarrhea, and protection from pathogen infection.
Research frontiers
Recent evidence suggest that the interaction of commensal bacteria and probiotics with the immune system is more than a mechanical engagement of bacterial cell wall components with immune cell receptors and includes an active cross-talk between live bacteria and the host through secreted substances (metabolites). This is an active area of research and data from microbiome genomic sequencing suggests that the majority of predicted genes encode proteins with unknown functions.
Innovations and breakthroughs
Most of the published work on probiotics interacting with the immune system has focused on the bacterial cell wall activating the immune system through engagement of the Toll-like receptor (TLR) family, in particular TLR2 and TLR4. Much less research has focused on secreted metabolites and very little is known about what these secreted compounds are. The data presented here showed that a primary mechanism of action of Ganeden Bacillus coagulans 30 (GBC30) metabolites involved support of more mature phenotypes of antigen-presenting cells, important for immunological decision-making. An immature antigen presenting cell may fail in triggering an appropriate immune defense reaction, while either inducing immunological unresponsiveness (anergy) towards the antigen, or induce an allergic reaction to the antigen.
Applications
The support of antigen-presenting cells in vitro by GBC30 metabolites suggests that consumption of GBC30 may lead to in vivo effects of improved decision-making in the gut-associated lymphoid tissue (GALT), translating into clinical observations of improved immunity against infections, and reduced immunological anergy and allergy.
Terminology
Cluster of differentiation 14 is a monocyte marker and functions as a co-receptor for bacterial lipopolysaccharide recognition. It is highly expressed on the cell surface of monocytes and macrophages; GALT is a mucosa-associated lymphoid tissue lining the gastrointestinal tract from the esophagus to the colon. It contains immune cells and plays an important part in preventing the immune system from reacting to the resident microflora as well as defence from pathogens; Anergy refers to the absence of a normal immune response to a specific antigen or allergen.
Peer review
The authors present a paper that aimed to investigate any differences between the effects of a crude preparation of GBC30 bacterial culture metabolites compared to the fractionated preparations on the maturation of peripheral blood mononuclear cell. It demonstrates that probiotic bacteria produce metabolites that activate cells of the immune system, beyond what is expected from simple bacterial cell wall components.
Footnotes
Supported by A Research Sponsorship from Ganeden Biotech, Ohio, United States
Peer reviewer: Ian C Lawrance, MB, BS (Hons), PhD, FRACP, Professor, Director, Centre for Inflammatory Bowel Disease, School of Medicine and Pharmacology, University of Western Australia, Centre for Inflammatory Bowel Disease, Fremantle Hospital, T Block, Alma Street, Fremantle WA 6160, Australia
S- Editor Shi ZF L- Editor A E- Editor Zhang DN
References
- 1.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 3.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–577. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 4.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endres JR, Qureshi I. Probiotics for symptoms of IBS: A review of controlled trials. Nat Med J. 2009;1:1–5. [Google Scholar]
- 7.Endres JR, Clewell A, Jade KA, Farber T, Hauswirth J, Schauss AG. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem Toxicol. 2009;47:1231–1238. doi: 10.1016/j.fct.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 9.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 10.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci. 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson KE, Weinstock GM, Highlander SK, Worley KC, Creasy HH, Wortman JR, Rusch DB, Mitreva M, Sodergren E, Chinwalla AT, et al. A catalog of reference genomes from the human microbiome. Science. 2010;328:994–999. doi: 10.1126/science.1183605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen GS, Benson KF, Carter SG, Endres JR. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010;11:15. doi: 10.1186/1471-2172-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen GS, Redman KA, Benson KF, Carter SG, Mitzner MA, Reeves S, Robinson L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: results of a placebo-controlled double-blinded crossover pilot study. J Med Food. 2011;14:1002–1010. doi: 10.1089/jmf.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 16.Ho CS, Munster D, Pyke CM, Hart DN, López JA. Spontaneous generation and survival of blood dendritic cells in mononuclear cell culture without exogenous cytokines. Blood. 2002;99:2897–2904. doi: 10.1182/blood.v99.8.2897. [DOI] [PubMed] [Google Scholar]
- 17.Foligne B, Zoumpopoulou G, Dewulf J, Ben Younes A, Chareyre F, Sirard JC, Pot B, Grangette C. A key role of dendritic cells in probiotic functionality. PLoS One. 2007;2:e313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzello V, Bonaccorsi I, Dongarrà ML, Fink LN, Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J Biomed Biotechnol. 2011;2011:473097. doi: 10.1155/2011/473097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kvistborg P, Boegh M, Pedersen AW, Claesson MH, Zocca MB. Fast generation of dendritic cells. Cell Immunol. 2009;260:56–62. doi: 10.1016/j.cellimm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Ge W, Yang J, Vladau C, Suzuki M, Chen D, et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol. 2007;178:5480–5487. doi: 10.4049/jimmunol.178.9.5480. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 23.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, Suzuki A, Sata M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417–426. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, Velge-Roussel F, Lebranchu Y. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008;3:e2753. doi: 10.1371/journal.pone.0002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156:78–87. doi: 10.1111/j.1365-2249.2009.03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeuthen LH, Fink LN, Frøkiaer H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology. 2008;124:489–502. doi: 10.1111/j.1365-2567.2007.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, Pasco DS. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int Immunopharmacol. 2008;8:1023–1032. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol. 2009;31:655–659. doi: 10.1358/mf.2009.31.10.1441078. [DOI] [PubMed] [Google Scholar]