Fig. 1.
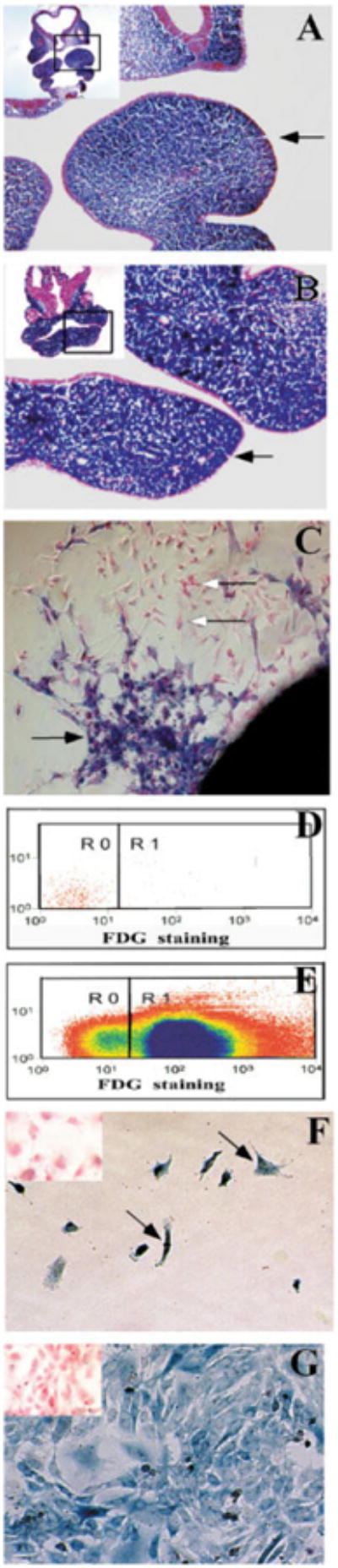
Establishing a pure population of post-migratory CNC cells in vitro. CNC-specific β-galactosidase activity is present in transverse sections of the first branchial arch from Wnt-cre/R26R mouse embryos at E10.5 (A) and E11.5 (B). The arrows indicate the first branchial arch. The position of each section in the embryo is framed out in the inlet. C: Cultured E9.0 neural tube explant (for one day) shows neural crest (blue, black arrow) and non-neural crest (pink, white arrow) cells migrate away from the explant. D: Fluorescence activated cell sorting (FACS) was used to separate CNC from non-CNC cells. Cells without FDG staining are used to establish a base line (R0) prior to cell sorting. E: Following FDG staining, FDG positive CNC cells (R1) are collected for cell culture study, while FDG negative cells are treated as non-CNC cells (R0). F: One day following cell sorting, CNC cells (arrow) have attached to the plate in culture. G: Five days following cell sorting, CNC cells have expanded and become confluent in the dish. All cells show CNC-specific β-galactosidase activity (blue). There is no non-CNC-derived cell in the culture dish. The cell cultures are counterstained with fast red. Insets (E,F): The non-CNC derived cells are pink in color following β-gal and fast red staining.
