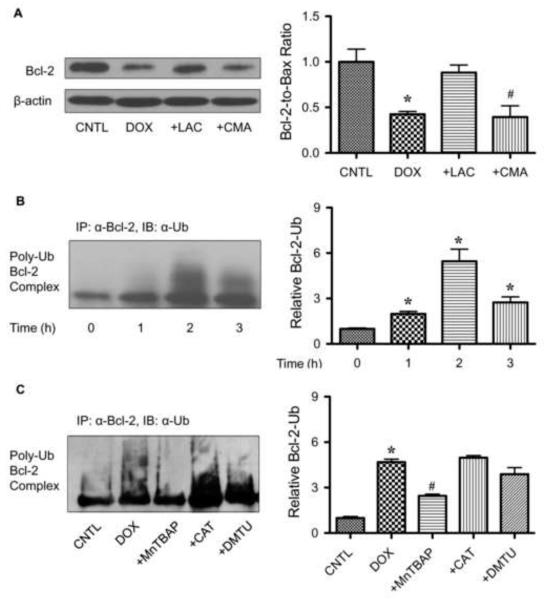
FIGURE 6.
Doxorubicin downregulates Bcl-2 through ubiquitin-proteasomal degradation. A, HaCaT cells were pretreated with the proteasome inhibitor lactacystin (LAC, 10 μM) or lysosome inhibitor concanamycin A (CMA, 1 μM) for 1 h, and then treated with DOX (1.5 μM) for 24 h. Bcl-2 expression was determined by Western blots using anti-Bcl-2 antibody. B, cells were pretreated with LAC (10 μM) for 1 h (to prevent proteasomal degradation of Bcl-2) and then treated with DOX (1.5 μM) for various times (0-3 h). Cell lysates were immunoprecipitated with anti-Bcl-2 antibody and the immune complexes were analyzed for ubiquitin by Western blotting. C, cells were pretreated with LAC (10 μM) for 1 h and then treated with DOX (1.5 μM) in the presence or absence of MnTBAP (50 μM), catalase (CAT, 7,500 units/ml), or DMTU (5 mM). Analysis of ubiquitin was performed as described at 2 h post-treatment where ubiquitination was found to be maximal. Plots are mean ± S.D. (n = 3). *, p < 0.05 versus non-treated control. #, p < 0.05 versus DOX-treated control.