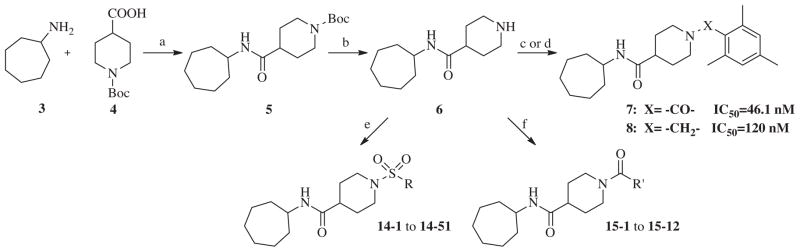
Scheme 1.
Reagents and conditions: (a) EDC, CH2Cl2, rt, 24 h, 68%; (b) TFA, CH2Cl2, rt, 24 h, 89%; (c) 2,4,6-trimethylbenzoyl chloride, Et3N, CH2Cl2, rt, 24 h, 85%; (d) mesitaldehyde, NaBH(OAc)3, CH2Cl2, rt, 12 h, 82%; (e) R-SO2Cl, Et3N, CH2Cl2, rt, 24 h, 70–91%; (f) R′OCl, Et3N, CH2Cl2, rt, 24 h, 85–92%.