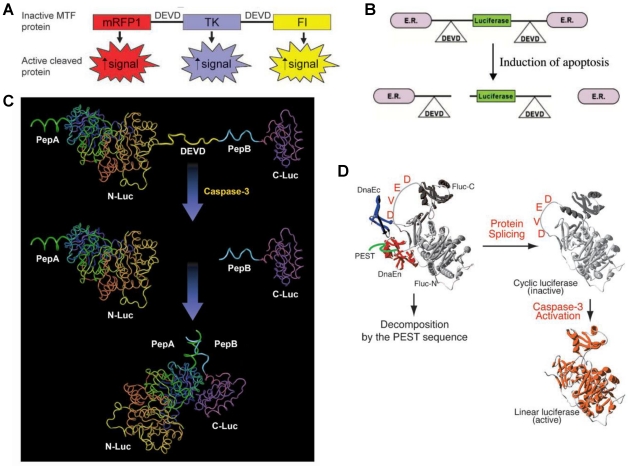
Figure 3.
Activatable reporter gene systems for imaging of caspase 3 activities. A. Activation of caspase-3 cleaves the MTF fusion protein into the three reporter protein components. Upon cleavage, the reporter proteins (mRFP1, FL, andTK) with attenuated activity in fused form (MTF) gain significantly higher activity and can be used for noninvasive imaging of apoptosis. Reproduced with permission from ref. 73. B. Chimeric polypeptides consisting of a reporter molecule fused to the ER resulted in silencing of the reporter activity. Inclusion of a protease cleavage site between these domains provided for protease-mediated activation of the reporter molecule after separation of the silencing domains (i.e., ER). Reproduced with permission from ref. 71. C. The ANLucBCLuc apoptosis imaging reporter constitutes the split luciferase (NLuc and CLuc) domains fused to interacting peptides, peptide A and peptide B, with an intervening caspase-3 cleavage motif. Upon induction of apoptosis, the reporter molecule is proteolytically cleaved by caspase-3 at the DEVD motif. This cleavage enables interaction between pepANLuc and pepBCLuc, thus reconstituting luciferase activity. Reproduced with permission from ref. 61 72. D. For an efficient protein splicing reaction, the C- and N-terminal fragments of DnaE (DnaEc and DnaEn, respectively) are connected with the N- and C-terminal ends of the circularly permuted luciferase. In addition, a PEST sequence was attached to the C-terminal end of the fusion construct to accelerate degradation of a protein. The PEST sequence results in the degradation of only unspliced products because cyclic Fluc does not possess the PEST sequence. Consequently, only cyclic Fluc accumulates inside the cells. If caspase-3 is activated in cells expressing the cyclic Fluc, the Fluc changes into an active form and its luminescence activity is restored. Thus, cells expressing the cyclic Fluc allow monitoring of caspase-3 activity with luminescence signals. Reproduced with permission from ref. 74