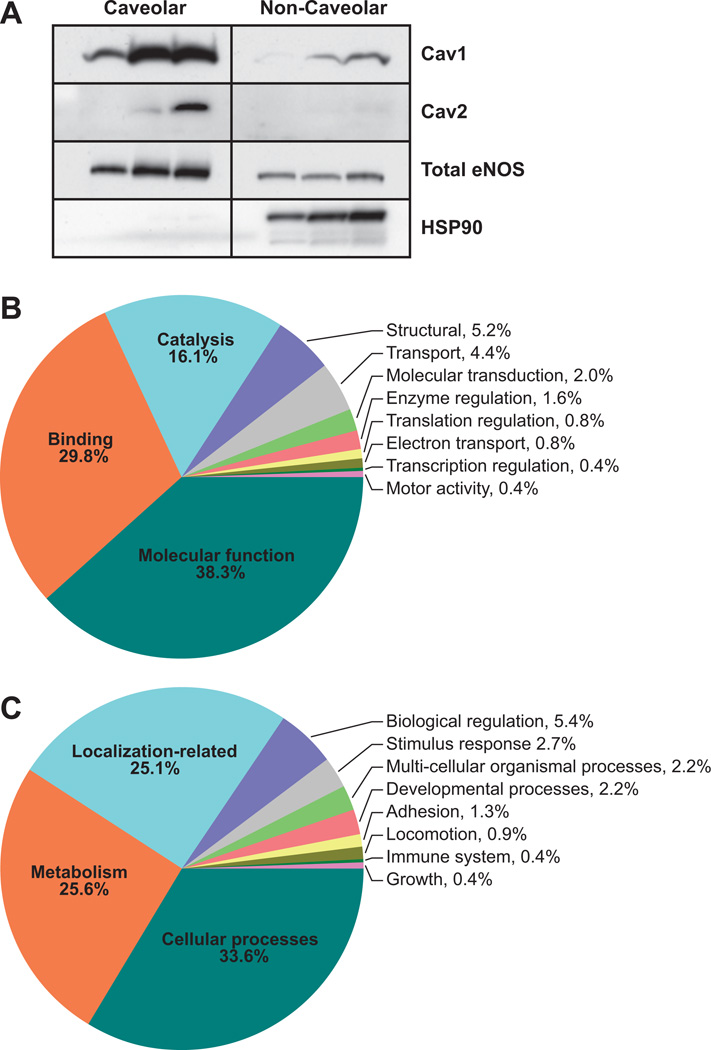
Figure 1.
A, Immunoblot validation of enriched caveolae from control endothelial cells shows the presence of the caveolar scaffolding protein cav-1 (optical density: caveolar, 0.802±0.295; noncaveolar, 0.0567±0.0383) and endothelial NO synthase (eNOS; optical density: caveolar, 0.725±0.173; noncaveolar, 0.155±0.0274) in the proteome (Table S1). Western blotting showed low expression of cav-2 (optical density: caveolar, 0.387±0.325; noncaveolar, 0.0543±0.00549), whereas heat shock protein (HSP) 90 (optical density: caveolar, 0.00633±0.00203; noncaveolar, 0.595±0.191) was barely detectable in the caveolar domain and was almost exclusively found only in the noncaveolar domain, confirming the proteomic observations that cav-2 and HSP90 were not detected among the 75 most enriched caveolar proteins that were examined in this study. B, Endothelial cells treated with or without ATP (100 µmol/L, 5 minutes) were used for caveolae isolation. The entire caveolar proteome with the Gene Ontology term listed in National Center for Biotechnology Information database was classified according to cellular processes. C, Proteins with Gene Ontology terms were classified according to biological processes.