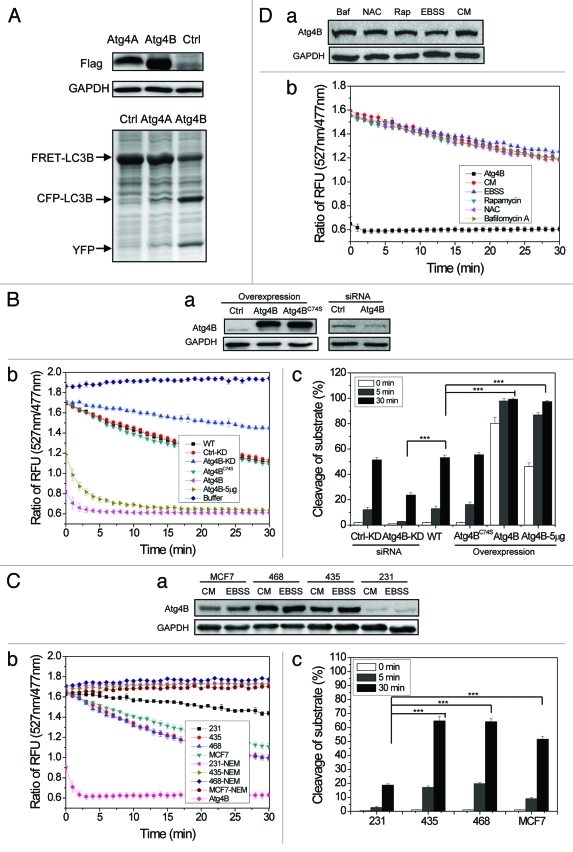
Figure 4. Measurements of Atg4B activity in cell lysates using the FRET-based assay. (A) HEK-293A cells were transfected with Flag-Atg4A, Flag-Atg4B or vector for 24 h. Cells were harvested and cell lysates (25 μg) were mixed with FRET-LC3B (5 µg) for 20 min before the reaction was stopped by the addition of sample loading buffer, and separated by SDS-PAGE. The expression level of Flag-Atg4A and Flag-Atg4B was detected by immunoblot assay using an anti-Flag antibody. The cleavage was assessed after CBB staining. (B) HEK-293A cells were transfected with Flag-Atg4B, Flag-Atg4BC74S or the control vectors. Alternatively, they were transfected with a control siRNA or a siRNA against Atg4B. Cell lysates (20 μg) were prepared and subjected to immunoblot analysis (a), or incubated with FRET-LC3B (3 µg) for the cleavage assay (b). Five micrograms (indicated) or 20 µg (all others) of lysates were used. WT, control; KD, control or Atg4B siRNA-treated. The percentage of substrate cleavage (c) was calculated. (C) Four breast cancer cell lines were cultured in complete medium (CM) or EBSS for 4 h. Cell lysates (20 μg) were prepared and subjected to immunoblot analysis (a), or incubated with FRET-LC3B for the cleavage assay (b). A positive control group with purified recombinant Atg4B (2 μg) was included. A generic cysteine protease inhibitor, NEM (100 µM), was included in some reactions as indicated. The percentage of substrate cleavage (c) was calculated. (D) HEK-293A cells were incubated in EBSS, or in complete medium alone (CM) or with rapamycin (Rap, 2 µM), N-acetylcysteine (NAC, 20 mM) or bafilomycin A1 (Baf, 1 µM) for 4 h. Cell lysates were prepared and subjected to immunoblot analysis (a), or incubated with FRET-LC3B for the cleavage assay (b). A positive control group with purified recombinant Atg4B (2 μg) was included (indicated as Atg4B). Data represent the mean ± SD from three independent experiments. ***p < 0.001 (one way ANOVA, B, c; and C, c).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.