Abstract
We aimed to assess the association between red blood cell distribution width (RDW) and mortality in patients enrolled by a Geriatric Department. One hundred twenty-two patients were followed up during 5 years. The primary end point was all-cause mortality, and hazard ratios were estimated using a Cox proportional hazard model. Higher RDW values were strongly associated with an increased risk of death. Survival curves across RDW quartiles were statistically different according to the log-rank test (p = 0.017). The first quartile presented higher probability of survival compared to the last one. The gradient from lower to higher risk across quartiles was clear both in the 5-year mortality risk and in the mortality rate per 100 person-years, which ranged from 18.9 to 42.6. However, in the Cox regression model after adjusting for age, severity, and other factors, excess risk was only observed in the highest RDW quartile, with a hazard ratio of 2.24 (CI95% 1.13–4.42) vs the first quartile. RDW is a good predictor of mortality in hospitalized older adults beyond those with cardiovascular risk factors, and it could serve as an integrative measure of multiple clinical and subclinical processes simultaneously occurring in complex patients.
Keywords: Aging, Red cell distribution width, Mortality, Risk factors
Keywords: Life Sciences, Molecular Medicine, Geriatrics/Gerontology, Cell Biology
Introduction
The red cell distribution width (RDW) is a measure of variability in the size of circulating erythrocytes with higher values reflecting greater heterogeneity in cell sizes (anisocytosis) and is part of a complete blood count (England and Down 1974). The RDW was initially considered to be a tool to distinguish microcytic anemia because of iron deficiency from that attributed to thalassemia, but recent studies have suggested that RDW has further clinical significance besides its possible utility in the evaluation of anemia. The RDW has been evaluated as a potential screening marker for colon cancer and celiac disease (Sategna et al. 2002; Spell et al. 2004), prognostic marker in heart failure (Felker et al. 2007; Förhécz et al. 2009; Allen et al. 2010; Hammarsten et al. 2010; van Kimmenade et al. 2010), coronary heart disease events (Dabbah et al. 2010; Tonelli et al. 2008; Zalawadiya et al. 2010), biomarker in rheumatoid arthritis (Lee and Kim 2010), associated with the metabolic syndrome (Sánchez-Chaparro et al. 2010), and mortality in patients with stroke (Ani and Ovbiagele 2009). It is also a consistent and strong predictor of total and cause-specific mortality in older adults in community-based samples (Perlstein et al. 2009; Patel et al. 2009, 2010). The mechanism is unclear, but this association is independent of several factors, including inflammation, anemia, nutritional status, and age-associated diseases (Patel et al. 2009). We are unaware of any studies that have looked at the prognostic value of RDW among patients enrolled by an Acute Geriatric Department and its impact on mortality outcomes. Given that the RDW is routinely reported by clinical laboratories as a component of the complete blood count without additional costs, understanding its prognostic significance could be very valuable for risk stratification in clinical decision making. In this study, we have aimed to assess the association of RDW with mortality among some of the patients habitually supervised by our department.
Methods
Study population
This is a prospective study derived from a cohort of 122 patients older than 75 years hospitalized in 2005 in the Hospital of Navarra (100 from an acute geriatric ward and 22 from and orthopedic ward). Patients were consecutively recruited for the study at admission time and then followed up during 5 years. The full clinical records of the patients were registered at inclusion with a detailed physical status. An investigator blinded to all other clinical and outcome data collected data on laboratory tests (including RDW). All the 120 patients were successfully followed up by means of visits, phone interviews, and the hospital’s medical registry (which is electronic).
Laboratory methods
Blood samples were taken after 6 h of fasting between 8 and 10 AM by antecubital venipuncture in the first 48 h of the admission. RDW was reported as the coefficient of variation (percentage) of red blood cell volume measured with an automated Beckman Coulter Conter (LH-750). The normal range for RDW in our laboratory is 11.5% to 14.5%. For subgroup analysis, anemia was defined as hemoglobin levels lower to 13 g/dL in men and 12 g/dL in women, in accordance with the World Health Organization (WHO) criteria.
Statistical analysis
The primary end point was all-cause mortality at 5 years. This variable was utilized as a dichotomized categorical variable (deceased vs. alive at 5 years). The values of RDW were first categorized into quartiles (Q1, <13.75; Q2, 13.75–14.65; Q3, 14.65–16.32; Q4, >16.32). Patient characteristics at baseline were summarized across quartiles using the mean and standard deviations for continuous normal variables, the median and interquartile ranges for continuous non-normal and ordinal variables, and frequencies and percentages for categorical variables. A standard set of variables that might confound the association of RDW with mortality included demographic and other factors (age, sex, B12 vitamins, folic acid, and age-associated medical conditions). We also included an innovative approach including several measures of complexity Charlson Index, number of diagnosis achieved after a complete evaluation of the patient, number of drugs, Geriatric Cumulative Illness Rating Scale (CIRS-G), and American Society of Anesthesiologists scale. Differences among patient characteristics across RDW quartiles were evaluated using ANOVA for continuous normal variables, Kruskal–Wallis test for continuous not normal variables, Kendall tau-b for ordinal variables, χ2 test for trend for dichotomous variables, and Fisher’s exact test for polychotomous variables. Survival functions across RDW quartiles were assessed with Kaplan–Meier curves and log-rank test. The 95% confidence intervals (CI95%) for mortality risks and mortality rates were derived with the continuity correction (Fleiss Quadratic) and the mid-P exact test, respectively. To adjust for multiple risk factors, a Cox proportional hazard model was fitted, including as exposure variable the quartiles of RDW and as covariates potential confounders (age, sex, hemoglobin levels, Barthel, and CIRS-G) and baseline characteristics that were found to be associated with RDW. An assessment of the RDW estimate and precision was used to decide to maintain or not the covariates in the model. All analyses used two-sided tests with an overall significance level of α = 0.05. All data analyses were conducted using SPSS (version 17) and R (version 2.9.2).
Results
Baseline characteristics description and association with RDW
Baseline characteristics are given stratified by quartiles of RDW in Table 1. The study population was very complex with a high number of diagnosis, drugs, and CIRS-G. The mean RDW was 16.5 ± 2.5% (median RDW 15.9%, interquartile range 14.7% to 18.1%). Participants with higher RDW values were more likely to have lower levels of hemoglobin (p = 0.006), anemia (WHO criteria; p = 0.002), congestive heart failure (p < 0.001), higher number of diagnostics (p = 0.001) and CIRS-G (p = 0.048), and higher values of creatinine (p = 0.033). We did not find an association with C-reactive protein, several analytic parameters, and other age-associated diseases. There was a higher prevalence of nutrient deficiencies in participants with higher RDW, although a clear gradient across RDW quartiles was not observed.
Table 1.
Baseline characteristics by quartiles of RDW
| Quartile 1 (<13.75) | Quartile 2 (13.75–14.65) | Quartile 3 (14.65–16.32) | Quartile 4 (>16.32) | Test | p | |
|---|---|---|---|---|---|---|
| Participants (n) | 30 | 31 | 31 | 30 | ||
| Agea | 83.73 (5.28) | 85.77 (6.43) | 86.84 (4.42) | 85.27 (4.88) | Aov F = 1.80 | 0.151 |
| Sexb | ||||||
| Females | 19 (63) | 16 (52) | 21 (68) | 13 (43) | χ2trend = 1.143 | 0.285 |
| Males | 11 (37) | 15 (48) | 10 (32) | 17 (57) | ||
| Hemoglobin (g/dL)c | 12.7 (11.1, 14.5) | 13.2 (11.1, 14.6) | 11.4 (10.2, 12.4) | 11.1 (9.5, 12.9) | KW = 12.344 | 0.006 |
| Anemiab | 11 (37) | 16 (52) | 20 (64) | 22 (73) | χ2trend = 9.255 | 0.002 |
| Creatinine (mg/dL)c | 1 (0.9–1.1) | 1 (0.8–1.5) | 1.2 (0.8–1.6) | 1.2 (0.9–1.7) | KW = 8.718 | 0.033 |
| CPR (mg/L)c | 93 (33–116) | 96 (23–182) | 22 (14–58) | 51 (17–106) | KW = 4.311 | 0.230 |
| B12 vitamin (pg/mL)c | 542 (405,598) | 523 (367,750) | 538 (310–738) | 465 (352–965) | KW = 0.585 | 0.900 |
| Leucocites (/mm3)c | 9.3 (7.4–11.3) | 8.1 (6.4–12.4) | 8.8 (4.8–12.4) | 8.2 (6.2–14.6) | KW = 1.412 | 0.703 |
| Folic acid (ng/mL)c | 5.9 (4.8–8.0) | 5.0 (4.2–8.3) | 5.0 (3.8–8.1) | 5.6 (4.1–7.7) | KW = 1.515 | 0.679 |
| Total protein (g/dL)a | 6.37 (0.73) | 6.10 (0.79) | 6.20 (0.81) | 6.06 (0.57) | Aov F = 0.952 | 0.418 |
| Cholesterol(mg/dL)a | 160.0 (53.0) | 158.9 (43.8) | 159.4 (43.9) | 150.1 (43.1) | Aov F = 0.293 | 0.831 |
| Cardiac failureb | 5 (17) | 9 (29) | 16 (52) | 17 (57) | χtrend2 = 12.97 | <0.001 |
| COPDb | 5 (17) | 2 (7) | 7 (23) | 9 (30) | χtrend2 = 3.138 | 0.076 |
| Dementiab | 8 (27) | 11 (35) | 9 (29) | 10 (33) | χtrend2 = 0.124 | 0.725 |
| HTAb | 23 (77) | 16 (52) | 24 (77) | 26 (87) | χtrend2 = 2.433 | 0.119 |
| Diabetes mellitusb | 7 (23) | 9 (29) | 7 (23) | 4 (13) | χtrend2 = 1.166 | 0.280 |
| Cancer last 5 yearsb | 5 (17) | 7 (23) | 8 (26) | 9 (30) | χtrend2 = 1.549 | 0.213 |
| Barthel admissionc | 80 (64–100) | 60 (15–100) | 65 (20–90) | 90 (45–100) | KW = 6.993 | 0.072 |
| Ner diseasesc | 4.0 (3.0–6.3) | 6.0 (4.0–9.0) | 5.0 (4.0–8.0) | 9.0 (5.8–11) | KW = 15.510 | 0.001 |
| Ner of drugsc | 6.5 (3.0–8.3) | 5.0 (3.0–7.0) | 7.0 (4.0–8.0) | 7.0 (6.0–8.3) | KW = 4.568 | 0.206 |
| Karnofsky’s indexc | 60 (50–80) | 50 (40–70) | 50 (40–60) | 60 (40–72) | KW = 6.707 | 0.082 |
| Charlson Indexc | 1.0 (0.0–3.0) | 2.0 (0.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | KW = 2.236 | 0.525 |
| ASA indexc | 3.0 (2.0–3.0) | 4.0 (2.0–4.0) | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) | KW = 5.999 | 0.112 |
| CIRS-Gc | 20 (17–23) | 22 (19–26) | 21 (18–23) | 22.5 (19.8–25.5) | KW = 7.915 | 0.048 |
Aov F analysis of variance F statistics, χ2trend test for trend in proportions, KW Kruskal–Wallis test, CRP C-reactive protein, COPD chronic obstructive pulmonary disease, HTA hypertension, CIRS-G Geriatric Cumulative Illness Rating Scale, ASA American Society of Anesthesiologists
aMean (standard deviation)
bNer of cases (%)
cMedian (interquartile range)
Mortality risk across RDW quartiles
In the 5-year follow-up period, 80 of the 122 patients (65.6%) died. The mortality rates and mortality risks for the whole period show a gradient from lower to higher risk across RDW quartiles, with rates ranging from 18.9 to 42.6 and risks from 0.60 to 0.83 (Table 2).
Table 2.
Mortality risks and rates by RDW quartile
| RDW by quartile | Deaths | 5-year mortality risk (CI95%) | Mortality rate (CI95%) per 100 person-years |
|---|---|---|---|
| Q1 (<13.75) | 18 | 0.60 (0.41, 0.77) | 18.92 (11.57, 29.33) |
| Q2 (13.75–14.65) | 23 | 0.74 (0.55, 0.87) | 28.89 (18.32, 43.36) |
| Q3 (14.65–16.32) | 24 | 0.77 (0.58, 0.90) | 28.99 (18.58, 43.14) |
| Q4 (>16.32) | 25 | 0.83 (0.65, 0.94) | 42.62 (28.19, 61.98) |
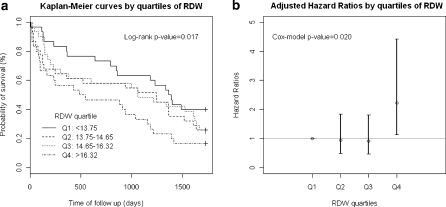
Survival curves across RDW quartiles were statistically different according to the log-rank test (p = 0.017). The first quartile presents higher probability of survival compared to the rest of quartiles, with no apparent differences between the intermediate ones (Fig. 1a). In the Cox model, adjusting for age, sex, hemoglobin, Barthel on admission, creatinine, CIRS-G, and cardiac failure, the association between RDW and mortality risk remains significant (p = 0.020), but the excess risk is only observed in the highest RDW quartile, with a hazard ratio of 2.24 (CI95% 1.13–4.42), compared to the first quartile (Fig. 1b). Higher values of creatinine and age and lower values of Barthel index at admission are associated with higher mortality risk (Table 3).
Fig. 1.
a Kaplan–Meier curves by quartiles of RDW. b Adjusted hazard rations by quartiles of RDW
Table 3.
Cox regression results modeling mortality by RDW quartile
| Variables in the model | Adjusted HR (CI95%) | p value |
|---|---|---|
| RD W by quartile | 0.020 | |
| Q1 (<13.75) | Reference | |
| Q2 (13.75–14.65) | 0.95 (0.49, 1.84) | |
| Q3 (14.65–16.32) | 0.92 (0.47, 1.82) | |
| Q4 (>16.32) | 2.24 (1.13, 4.42) | |
| Sex | 0.186 | |
| Females | Reference | |
| Males | 0.72 (0.46, 1.16) | |
| Age | 1.08 (1.04, 1.12) | <0.001 |
| Hemoglobin | 1.02 (0.91, 1.14) | 0.759 |
| Cardiac failure | 0.76 (0.56, 1.51) | 0.740 |
| Creatinine | 1.38 (1.03, 1.86) | 0.034 |
| CIRS-G | 0.98 (0.94, 1.03) | 0.432 |
| Barthel | 0.99 (0.98, 0.99) | <0.001 |
Discussion
The principal finding of our study is that greater red blood cell size heterogeneity, as reflected by higher RDW, is associated with higher mortality risks in geriatric hospitalized patients. Increased RDW is usually associated with ineffective red cell production or hemolysis but has recently been associated with adverse outcomes in cardiovascular and noncardiovascular disease states (Tonelli et al. 2008; Perlstein et al. 2009; Patel et al. 2009, 2010; Hampole et al. 2009). The magnitude of the risk associated with higher RDW derived in this study agrees with the findings of previous studies. This association remained significant even after adjustment for a wide variety of clinically relevant covariates. These covariates included not only important laboratory and clinical parameters but also measures of comorbidity and function (Barthel Index) that are not included in previous studies and are very important from the geriatric point of view. Supporting the validity of our findings, other independent prognostic markers for survival that have been previously accepted included the presence of heart failure (Felker et al. 2007; Allen et al. 2010; Förhécz et al. 2009, 2010; van Kimmenade et al. 2010; Hammarsten et al. 2010), kidney function (Lippi et al. 2008), and anemia, one of the most under-diagnosed disease in the elderly (Urrutia et al. 2010). Despite the well-known and established association between anemia and adverse outcomes in different patient populations, we do not believe that our findings were confounded by anemia. Not only was the association between RDW and mortality unaffected after adjustment for hemoglobin levels but also additional analyses restricted to patients who were not anemic demonstrated that RDW was still predictor of mortality in this subpopulation of patients. These findings are consistent with other studies (Perlstein et al. 2009; Patel et al. 2009, 2010) and add to growing data suggesting that RDW may be and independent predictor of several measures (Spell et al. 2004; Lippi et al. 2009).
To our knowledge, this is the first study that specifically looks at the impact of this parameter among hospitalized patients admitted in an Acute Geriatric Department considering also the impact of comorbidity and function. There is a work (Kho et al. 2007) in which the presence of nucleated red blood cells, burr cells, or absolute lymphocytosis at admission was each independently associated with a 3-fold increase in risk of death within 30 days since admission. Most of the previous research was mainly performed in patients with significant cardiovascular disease (Felker et al. 2007; Tonelli et al. 2008; Förhécz et al. 2009, 2010; Pascual-Figal et al. 2009; Allen et al. 2010; van Kimmenade et al. 2010; Hammarsten et al. 2010; Zalawadiya et al. 2010; Dabbah et al. 2010). Some measures that reflect that geriatric population is specifically complex and different to others are the number of diseases quantified or the CIRS-G. We found more useful these measures in that they reflect such complexity than the established Charlson Index, as we can see in Table 1. In fact, unlike other studies (except with pulmonary hypertension; Hampole et al. 2009), in our patients the RDW values were higher, suggesting that complexity of these patients in addition to age and the disease burden factor (Patel et al. 2009, 2010) may contribute to higher RDW values.
In our work, higher RDW levels across the different quartiles were associated with a higher number of patients with heart failure, but the association of heart failure with death was not statistically significant in the Cox model including RDW and other covariants. This suggests that RDW has clinical and subclinical implications beyond cardiovascular risk factors and that it may be a predictor in geriatric populations and not only in patients with cardiovascular disease.
After adjusting for confounders, higher levels of RDW were associated with higher risk of death, without showing a gradient across quartiles. With respect to the lowest quartile, we only found a significant risk increment in the highest quartile of RDW, i.e., in patients with RDW higher than 16.3, which could also be suggesting a different behavior of the indicator in acute geriatric patients, who could have higher RDW levels than other populations.
The biological mechanisms underlying the association of higher RDW with mortality risk is unclear. Increased anisocytosis is most commonly thought of as a consequence of anemia or anemia-related nutrient deficiency, but it is unlikely that anisocytosis itself is a causal factor in risk. RDW is frequently higher in situations of impaired red cell generation like iron, vitamin B12 deficiency, or folate deficiency, among others, suggesting that higher RDW may be an integrated risk factor for overall sub-optimal health status indicating diminished capacity for systemic repair, recovery, and defense (Zalawadiya et al. 2010). Identification of a putative mechanism is difficult because of the lack of epidemiological studies demonstrating factors that are associated with anisocytosis. A variety of mechanisms have been proposed for the association between RDW and mortality. Chronic subclinical inflammation could play a role (Libby et al. 2002). Inflammation may alter red blood cell circulation half-life, erythropoiesis, and red blood cell membrane deformability, factors that might lead to a more mixed population of RBC volumes in the circulation (Weiss and Goodnough 2005). Furthermore, it is believed that the increased prevalence of anemia with advancing age is due, in part, to the effects of proinflammatory cytokines on inhibiting the proliferation of erythroid progenitor cells and downregulating erythropoietin receptor expression. In our study, we did not find association of RDW with C-reactive protein (CRP) level, but most of the patients had high CRP when admitted to the Acute Geriatric Department. The association of RDW with mortality risk may, in part, be due to its association with prevalent disease in the acute setting. Previous studies showed that decreased serum antioxidant levels were also associated with increased RDW (Patel et al. 2009) and that serum antioxidants and inflammation predict red cell distribution width in older women (Semba et al. 2010). Exposure to greater oxidative stress might be yet another potential contributing mechanism. In patients with conditions characterized by increased levels of oxidative stress, such Down syndrome, poor pulmonary function, and dialysis (Ershler et al. 2005) RDW values are elevated. While erythrocytes have antioxidant capacity, they are prone to oxidative damage that reduces cell survival. In a population-based study, higher RDW values were independently associated with poorer pulmonary function, a condition associated with oxidative stress (Grant et al. 2003). Although more mechanistic research is needed, these studies indicate that RDW is associated with oxidative stress, possibly through increased red cells turnover.
Our study has several limitations that should be considered. Some of the HRs have wide confidence intervals (low precision) due to the limited number of patients. Laboratory assessments were made on a single occasion instead of serial measurements, and therefore, fluctuations in the CBC could not be evaluated. Increasing RDW during hospital course may reflect bone marrow response to the cumulative influences of multiple humoral mediators in the setting of an acute stress situation. Serial measurements of RDW would allow better characterization of the associations of RDW with mortality risk. We did not determine the cause of death to assess whether elevated RDW was associated with specific causes of death.
RDW could be an integrative biomarker that reflects dysfunction and impairment across multiple physiological systems related to the aging process or it could be caused by inflammation or age-associated diseases. It may serve as a measure of multiple processes simultaneously occurring in complex patients such as those controlled by a Geriatric Department. The identification of integrative prognostic markers may detect at-risk patients early, even at subclinical level, and as RDW is associated with multiple causes of death, it may provide insight into therapeutic approaches for patients with several diseases. An association of RDW with an occult disease may, in part, be responsible for the findings of this research. Some studies have already shown that RDW might be a useful screening marker (Sategna et al. 2002; Spell et al. 2004; Lippi et al. 2009).
It is unknown if the risk associated with RDW is modifiable or if RDW itself is modified by current therapies that alter prognosis. Further studies are needed to determine if or how this observation could guidance practice.
Conclusion
In summary, RDW is a good predictor of mortality in hospitalized older adults, and it could serve as a measure of multiple processes simultaneously occurring in complex patients.
References
- Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF., Jr Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16:230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. doi: 10.1016/j.jns.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105(3):312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- England JM, Down MC. Red-cell-volume distribution curves and the measurement of anisocytosis. Lancet. 1974;20:701–703. doi: 10.1016/S0140-6736(74)92904-3. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Sheng S, McKelvey J, Artz AS, Denduluri N, Tecson J, Taub DD, Brant LJ, Ferrucci L, Longo DL. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53:1360–1365. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659–666. doi: 10.1016/j.ahj.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width: a powerful prognostic marker in heart failure. Eur J Heart Fail. 2010;12:415. doi: 10.1093/eurjhf/hfq018. [DOI] [PubMed] [Google Scholar]
- Grant BJ, Kudalkar DP, Muti P, McCann SE, Trevisan M, Freudenheim JL, Schünemann HJ. Relation between lung function and RBC distribution width in a population study. Chest. 2003;124:494–500. doi: 10.1378/chest.124.2.494. [DOI] [PubMed] [Google Scholar]
- Hammarsten O, Jacobsson S, Fu M. Red cell distribution width in chronic heart failure: a new independent marker for prognosis? Eur J Heart Fail. 2010;12:213–214. doi: 10.1093/eurjhf/hfp208. [DOI] [PubMed] [Google Scholar]
- Hampole CV, Mehrotra AK, Thenappan T, Gomberg-Maitland M, Shah SJ. Usefulness of red cell distribution width as a prognostic marker in pulmonary hypertension. Am J Cardiol. 2009;104:868–872. doi: 10.1016/j.amjcard.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Kho AN, Hui S, Kesterson JG, McDonald CJ. Which observations from the complete blood cell count predict mortality for hospitalized patients? J Hosp Med. 2007;2:5–12. doi: 10.1002/jhm.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Arch Pathol Lab Med. 2010;134:505–506. doi: 10.5858/134.4.505.c. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest. 2008;68:745–748. doi: 10.1080/00365510802213550. [DOI] [PubMed] [Google Scholar]
- Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Franchini M, Guidi GC, Targher G. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47:353–357. doi: 10.1515/CCLM.2009.066. [DOI] [PubMed] [Google Scholar]
- Pascual-Figal DA, Bonaque JC, Redondo B, Caro C, Manzano-Fernandez S, Sánchez-Mas J, Garrido IP, Valdes M. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11(9):840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, Bandinelli S, Phillips CS, Yu B, Connelly S, Shlipak MG, Chaves PH, Launer LJ, Ershler WB, Harris TB, Longo DL, Guralnik JM. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;69:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Chaparro MA, Calvo-Bonacho E, González-Quintela A, Cabrera M, Sáinz JC, Fernández-Labandera C, Aguado LQ, Meseguer AF, Valdivielso P, Román-García J. Higher red blood cell distribution width is associated with the metabolic syndrome: results of the Ibermutuamur Cardiovascular Risk assessment study. Diabetes Care. 2010;33:e40. doi: 10.2337/dc09-1707. [DOI] [PubMed] [Google Scholar]
- Sategna Guidetti C, Scaglione N, Martini S. Red cell distribution width as a marker of coeliac disease: a prospective study. Eur J Gastroenterol Hepatol. 2002;14:177–181. doi: 10.1097/00042737-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, Fried LP. Serum antioxidants and inflammation predict red cell distribution width in older women: The Women’s Health and Aging Study I. Clin Nutr. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell DW, Jones DV, Jr, Harper WF, Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28:37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M, Cholesterol and Recurrent Events (CARE) Trial Investigators Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- Urrutia A, Sacanella E, Mascaro J, Formiga F. Anemia en el anciano. Rev Esp Geriatr Gerontol. 2010;45:291–297. doi: 10.1016/j.regg.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Kimmenade RR, Mohammed AA, Uthamalingam S, Meer P, Felker GM, Januzzi JL., Jr Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Fail. 2010;12(2):129–136. doi: 10.1093/eurjhf/hfp179. [DOI] [PubMed] [Google Scholar]
- Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- Zalawadiya SK, Veeranna V, Niraj A, Pradhan J, Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010;106:988–993. doi: 10.1016/j.amjcard.2010.06.006. [DOI] [PubMed] [Google Scholar]