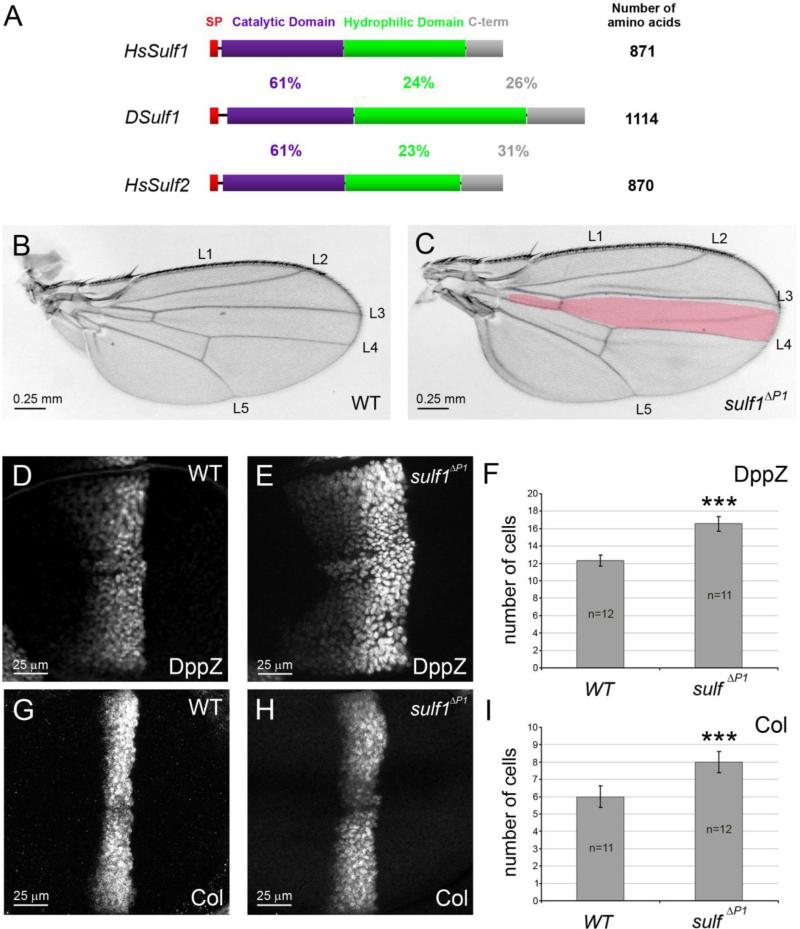
Figure 1. Dsulf1 loss-of-function allele impairs AP patterning of the wing by modulating Hh signalling.
(A) Schematic representation and alignment of Drosophila and Human Sulf proteins named DSulf1 and HsSulf1/HsSulf2, respectively. The size of each protein is indicated on the right. The region corresponding to the signal peptide (SP) is in red, the catalytic domain in purple, the hydrophilic domain in green and the C-terminal domain (C-term) in grey. Percentages of identity between DSulf1 and HsSulf1 or HsSulf2 are indicated for each conserved structural domain. (B, C) Here, as in all subsequent panels, adult wings are oriented with anterior to the top and distal to the right and the wild-type L3-L4 intervein domain, represented in red, is superimposed to the mutant wing. In adult Dsulf1ΔP1 mutants (C), the global size of the wing is enhanced and the L3-L4 intervein domain is enlarged (35±2 trichomes, C) compared to wild type (30±1, B). (D-H) Detection of Hh target gene expression in wt (D, G) and Dsulf1ΔP1 (E, H) 3rd instar wing discs oriented, in this figure and all the following ones, with anterior to the left, posterior to the right, dorsal up and ventral down. Magnification is indicated by scale bar in each figure. Note the enlargement of dpp-lacZ (dppZ, E) and Collier (Col, H) domains of expression in Dsulf1ΔP1 compared to wt discs (D, G). (F, I) Number of cell rows expressing dppZ (F) and Col (I) in wt and Dsulf1ΔP1 wing discs. Error bars represent the standard deviation (***=P<0.0005 using a t-test).