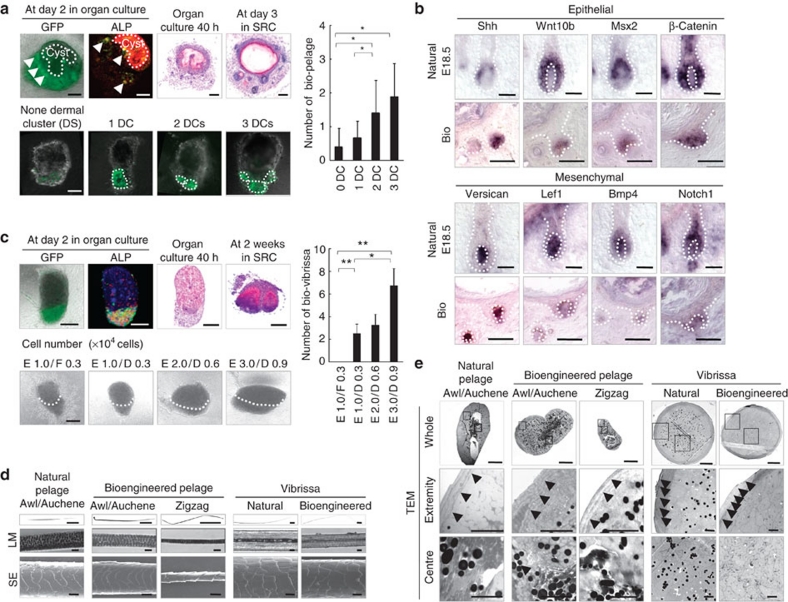
Figure 3. Control of the number and features of the bioengineered hairs.
(a) Analysis of EGFP fluorescence (green), ALP activity (red) and H&E staining in bioengineered pelage follicles reconstituted from normal epithelial cells and EGFP-labelled mesenchymal cells derived from embryonic skin at 2 days in organ culture (upper left) and subsequently transplanted into the subrenal capsule (SRC, upper left). Dermal cell clusters (DCs) in the bioengineered pelage germ were detected by EGFP fluorescence under confocal imaging (lower left). The number of bioengineered pelage follicles regenerated in vivo from the bioengineered hair follicle germ is shown in the lower left panels. The data are shown as the mean±s.d. (n>9). P<0.01 (*) and P<0.05 (**), analysed by t-test. Scale bars: 200 μm for H&E, 50 μm for immunofluorescent microscopy images, 1 mm for macroscopic images. (b) In situ hybridization analysis of the expression of regulatory genes during folliculogenesis in the natural and bioengineered pelage follicle in vivo. Dotted lines indicate the epithelial–mesenchymal boundary. Scale bars: 200 μm. (c) Analyses of EGFP fluorescence, enzymatic staining of ALP and H&E staining in adult vibrissa-derived bioengineered hair germ after 2 days in organ culture (upper left). Bioengineered vibrissa follicles regenerated in SRC for 2 weeks were also analysed using H&E staining. The bioengineered vibrissa germs were reconstituted using bulge epithelial cells (E) and primary culture DP cells (D) with various cell numbers (lower left), and murine sole skin dermis-derived fibroblasts (F) were used as a control for DP cells. The dotted lines in the lower left panels indicate the epithelial–mesenchymal boundary. The number of bioengineered vibrissa follicles regenerated from the in vivo transplantation of bioengineered hair follicle germ reconstituted under various conditions is described in the lower left. Data are shown as the mean±s.d. (n>4). P<0.01 (*) and P<0.05 (**) analysed by t-test. Scale bars, 100 μm. (d and e) Microscopic observation of the bioengineered pelage and vibrissa shafts. The hair shafts were analysed by using light microscopy (LM), SEM and TEM. Whole (TEM, upper), high-magnification extremity (middle), and central regions of the hair shaft (lower) are shown. Cuticle layers (arrowheads) and dense medullary granules (arrow) are shown in the fine TEM photographs of the extremity and centre regions. Scale bars, 1 mm for low magnification (LM), 20 μm for high magnification of LM and SEM, 10 μm for whole TEM and 2 μm for fine TEM.