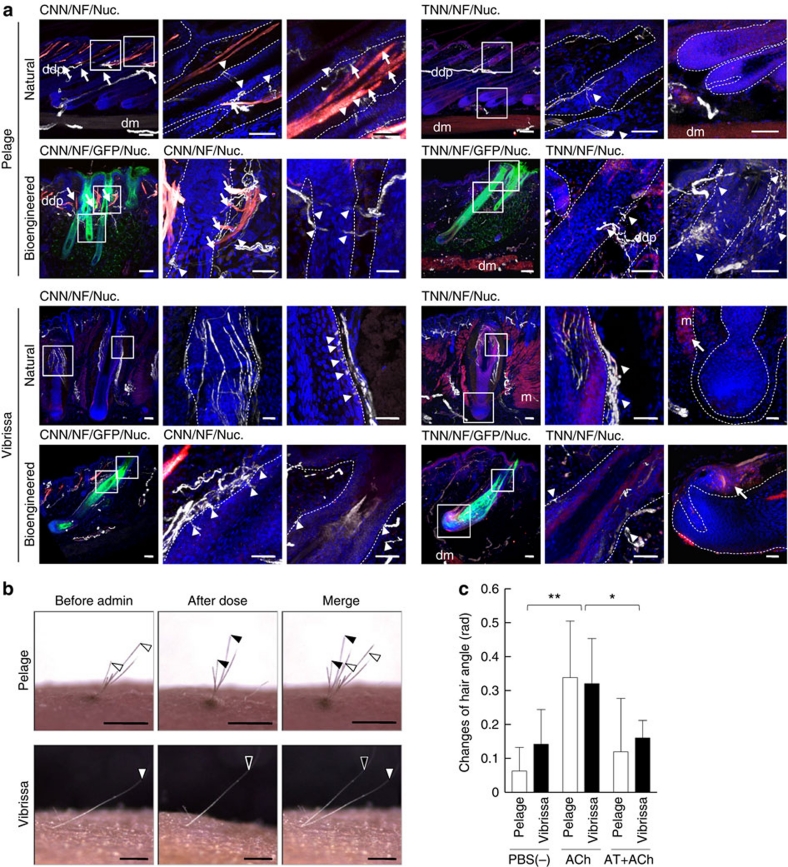
Figure 6. Analyses of the connections to surrounding tissues and the piloerection capability of bioengineered hair follicles.
(a) The connections of the follicles of a natural and EGFP-labelled bioengineered pelage (upper two lines) and natural and EGFP-labelled bioengineered vibrissa (lower two lines) to arrector pili muscles and nerve fibres were analysed by immunohistochemical staining using specific antibodies against calponin for smooth muscle (CNN, red in the left three panels), troponin for striated muscle (TNN, red in the right three panels) and neurofilament H (NF, white). The nuclei were stained using Hoechst 33258 (Nuc, blue). The boxed areas in the left panel in each data set are shown at a higher magnification in the right two panels. The arrows and arrowheads indicate the muscle and nerve fibres connected with pelage follicles, respectively. Broken lines indicate the outermost limit of the hair follicle. Scale bars, 100 μm in low-magnification and 50 μm in high-magnification photographs. ddp, deep dermal plexus; dm, dermal muscle layer; m, muscle. (b) Analysis of the piloerection capability of bioengineered hair follicles following an intradermal injection of ACh. The positions of the hair shafts (black or white arrowheads in the left) moved after this treatment (centre) and merged (right). Scale bars, 1.0 mm. (c) Assessment of hair angle changes associated with the bioengineered pelage (light bars) and vibrissae (dark bars) before and after the administration of ACh, without or with atropine (AT+ACh). The data are shown as the mean±s.e.; n=10 for the bioengineered pelage and n=6 for the bioengineered vibrissa. P<0.01 (*) and P<0.05 (**) were defined as statistically significant, as analysed by t-test.