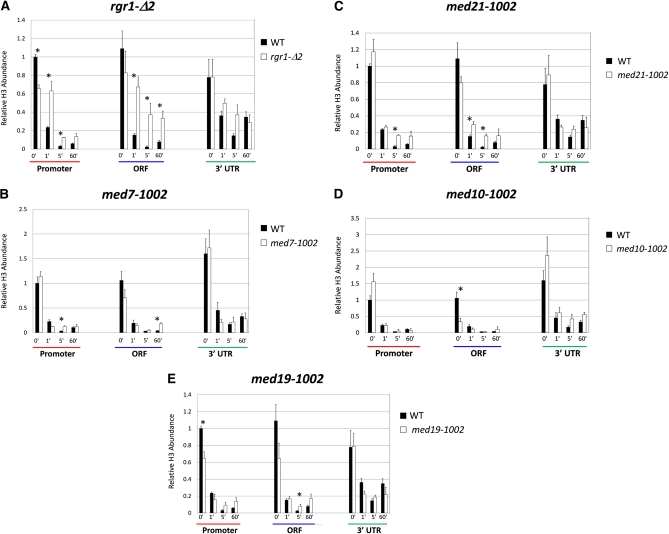
Figure 4 .
Mutations in the Med14/Rgr1 and Med21 subunits of Mediator diminish the extent of histone displacement at HSP82. Kinetics of histone H3 depletion over the promoter, ORF, and 3′-UTR of HSP82 in WT and mutant cells as assayed by ChIP–qPCR at the indicated times following an instantaneous 30° to 39° temperature shift. H3 globular domain abundance at HSP82 was normalized to its abundance at the PHO5 promoter. This was determined by dividing each HSP82 ChIP value (net nanograms DNA immunoprecipitated) by the corresponding PHO5 ChIP value derived from the same biological sample. Note that H3 occupancy of the PHO5 promoter is independent of both heat-shock and ewe strain background (data not shown and Sekinger and Gross 2001). Depicted are means ± SEM (N = 2; qPCR = 4). Asterisks signify a potentially significant difference between the indicated values (P ≤ 0.06; two-tailed t-test; equal variance). (A–E) H3 occupancy in MED+ and the indicated ewe mutant strains. A, C, and E represent one set of immunoprecipitation reactions; B and D represent a second, independently conducted set.