Summary
Background and objectives
Mechanical failure of the peritoneal dialysis (PD) catheter is an important cause of technique failure. Fluoroscopic guidewire manipulation may be undertaken in an attempt to correct the failure. The purpose of this study was to determine the efficacy of fluoroscopic manipulation of previously embedded PD catheters, the factors associated with successful manipulation, and the complication rate associated with manipulation.
Design, setting, participants, & measurements
A single-center, retrospective review of 70 consecutive PD patients undergoing fluoroscopic manipulation for mechanical failure of their PD catheter from June 2006 to February 2011 was undertaken. Logistic regression models were developed to determine the variables associated with successful manipulation.
Results
Of the 70 manipulations, 44 were successful (62.9%). In univariate analysis, catheters located in the pelvis compared with those in the upper abdomen (73.5% versus 42.9%, P=0.01) and catheters that were previously functional compared with those that failed at exteriorization (75.0% versus 46.7%, P=0.04) were more likely to be successfully manipulated. Time embedded, previous hemodialysis, and number of intra-abdominal surgeries were not correlated with likelihood of successful manipulation. In multivariate analysis, catheters located in the pelvis (P=0.01) and those with secondary failure (P=0.01) were more likely to successfully manipulated. Two of the patients developed peritonitis (2.9%), neither requiring cessation of PD.
Conclusions
Fluoroscopic manipulation is an effective and safe therapy for failed PD catheters that are unresponsive to conservative treatment. Properly positioned catheters and those that were previously functional are more likely to be successfully manipulated.
Introduction
Mechanical failure of the peritoneal dialysis (PD) catheter is the third leading cause of technique failure among prevalent PD patients, and the leading cause among incident patients (1). The most common causes of mechanical obstruction resulting in catheter failure are omental wrapping, adhesions with or without migration out of the pelvis, and intraluminal obstruction due to fibrin or blood (2). Guidewire manipulation of the obstructed catheter under fluoroscopic guidance is undertaken in many centers although the technique remains controversial because outcomes are variable and the technique cannot correct the underlying anatomic abnormalities that lead to the malfunction in many cases (3).
Our center places embedded PD catheters several months before the anticipated need for PD (4). Our previously published results have shown an increase in the rate of mechanical obstruction to catheter flow among those catheters that are left buried for >5 months before use (5). Patients with obstructed catheters are treated with vigorous flushing, high-dose laxatives, and tissue plasminogen activator (tPA) if there is evidence for inflow or outflow obstruction. Despite this, some patients have persistent obstruction and it is our practice to attempt fluoroscopic manipulation of these catheters. Those who fail fluoroscopic manipulation usually undergo laparoscopic repositioning of their catheter.
We undertook this retrospective review of our large population of patients with fluoroscopic manipulations to evaluate the efficacy of fluoroscopic manipulation for previously embedded PD catheters, to determine the complication rate associated with these manipulations, and to determine which factors are associated with a successful fluoroscopic manipulation. Specifically, we were interested in determining if the likelihood of successful manipulation depended on radiographic location of the catheter, primary failure (e.g., at the exteriorization of catheter) versus secondary failure (e.g., after previously functioning), duration of time the catheter was embedded before use, history of hemodialysis at the time of catheter insertion, or, number of intra-abdominal surgeries.
Materials and Methods
After research ethics board approval, a retrospective chart review was conducted of all patients who underwent fluoroscopic manipulation of nonfunctional PD catheters between June 2006 and February 2011 in our tertiary care hospital. Hospital and nephrology electronic databases were used to identify all patients who had undergone at least one fluoroscopic catheter manipulation. Basic demographic information including age, sex, and cause of ESRD were recorded. Other collected variables included body mass index (BMI), a history of hemodialysis before converting to PD (subclinical bleeding secondary to heparin for hemodialysis might contribute to intraluminal obstruction from blood clots), time from catheter insertion to exteriorization, time from catheter exteriorization to mechanical failure, number of previous intra-abdominal operations, position of the catheter at the time of obstruction, and complications of manipulation.
All PD catheters at our institution at the time of this study were swan neck catheters with a coiled tip. They were inserted by general surgeons or urologists using a technique that leaves the exterior portion of the catheter buried under the skin regardless of the expected time from operation to catheter use (4). Refractory catheter obstruction was defined as poor outflow and/or inflow of dialysate such that PD could not be successfully performed, despite a treatment algorithm designed to restore function. All patients with inflow problems undergo vigorous flushing with heparinized saline with a push and pull technique. If the flushing is not successful, 7 mg of tPA (1 mg/ml) is instilled into the catheter followed by 1 mg every 15 minutes for another 3 mg and then left overnight. A repeat attempt to flush the catheter occurs after the overnight tPA dwell. If inflow obstruction persists, then a fluoroscopic manipulation is ordered. The approach to outflow obstruction involves flushing the catheter, use of high-dose laxatives, abdominal radiography, and then fluoroscopic manipulation for refractory cases.
Position of the catheter at the time of failure was determined from the abdominal-pelvic radiograph. Catheter position was defined by the location of the catheter tip. Catheters located in the true pelvis were considered well positioned, whereas catheters on the pelvic brim were considered lower abdominal and those above the pelvic brim upper abdominal. The radiographs were read by two independent physicians for the purposes of this study, with disagreements being read by a third independent physician. Fluoroscopic manipulation was performed by six different radiologists using slight variations of the same technique. A guidewire was passed through the catheter clearing any fibrin and straightening the coiled tip of the catheter, potentially freeing the catheter from any fibrin sheath or adhesions. If the catheter had migrated and required repositioning, then the guidewire was angulated before insertion into the catheter, which allowed the catheter to be repositioned into the true pelvis. Good inflow and outflow were confirmed by the radiologist before completion of the procedure. Prophylactic antibiotics were given to patients before all manipulations.
Our primary outcome was successful manipulation, which was defined as adequate catheter function to permit PD at 30 days. Complications of the manipulation were also recorded. Peritonitis was considered to be related to the manipulation if it occurred within 30 days of the procedure. Univariate and multivariate logistic regression were performed to examine potential variables that were associated with successful fluoroscopic manipulation. The included variables were as follows: catheter position, primary versus secondary failure, time catheter was embedded, whether a patient was on hemodialysis before PD, time on hemodialysis, and the number of previous intra-abdominal operations. Statistical significance was defined as P≤0.05. A Kaplan–Meier survival curve was plotted to determine time from successful manipulation to subsequent event (recurrent failure, end of PD for noncatheter-related reasons, or end of study).
Results
Patients and Outcomes
Of the 415 patients who started training in our home dialysis unit, 70 patients were identified who underwent fluoroscopic manipulation of their PD catheter for mechanical obstruction. The types of obstruction were classified as outflow (52 of 70), inflow (1 of 70), both inflow and outflow (15 of 70), and unclassified (2 of 70) at the time of referral for fluoroscopic manipulation. The patient demographic characteristics and causes of ESRD are shown in Tables 1 and 2, respectively. Mean patient age was 61.5±14.1 years, mean BMI was 28±6.2 kg, and 71% were male. Twenty-five of the patients had experience with hemodialysis before starting PD. The median time on hemodialysis before transfer to PD was 198 days (interquartile range, 124–256 days). Of the 18 patients who were on hemodialysis <1 year before transfer to PD, 6 patients started hemodialysis acutely in hospital and later decided to change their treatment modality. One patient’s PD catheter was damaged at the time of exteriorization and the patient was transferred to hemodialysis temporarily until a new catheter was inserted. The remaining 11 patients had been followed in the progressive renal insufficiency clinic with PD as their chosen modality but the catheter had not been placed before the development of ESRD. Only seven of the patients had been on hemodialysis for >1 year before transfer to PD. The reasons for transfer from hemodialysis after >1 year of dialysis included vascular access problems (n=2), delayed treatment of a large incision hernia preventing PD catheter placement (n=1), transfer from another program (n=1), and lifestyle (n=3). Median time from insertion to exteriorization of the buried catheter was 77 days (range, 26–3101). Median time from exteriorization to catheter obstruction was 22 days (range, 0–982).
Table 1.
Demographic and selected patient characteristics
| Value | |
|---|---|
| Patient characteristic | |
| age (yr) (mean ± SD) | 61.5±14.1 |
| male sex, n (%) | 50 (71) |
| hemodialysis before peritoneal dialysis, n (%) | 25 (36) |
| previous intra-abdominal operations, n | 1 (IQR, 0–1); (range, 0–4) |
| Time variable (d) | |
| time on hemodialysis before catheter exteriorization | 0 (IQR, 0–135); (range, 0–2163) |
| time from catheter insertion to exteriorization | 77 (IQR, 49–295); (range, 26–3101) |
| time from catheter exteriorization to failure | 22 (IQR, 0–125); (range, 0–982) |
| Catheter position | |
| pelvis | 34 (49.2) |
| lower abdomen | 21 (30.4) |
| upper abdomen | 14 (20.3) |
Table 2.
Causes of ESRD
| Cause | % |
|---|---|
| Diabetes mellitus type 1 | 8.6 |
| Diabetes mellitus type 2 | 31.4 |
| Polycystic kidney disease | 10.0 |
| IgA | 8.6 |
| Hypertension | 5.7 |
| Idiopathic FSGS | 5.7 |
| Systemic lupus erythematosus | 4.3 |
| Ischemia | 2.9 |
| Othera | 14.3 |
| Not stated | 8.6 |
Other causes include one patient each (1.4%) with renal artery stenosis due to fibromuscular dysplasia, idiopathic nephrotic syndrome, idiopathic GN, reflux nephritis, infective endocarditis, scleroderma, chronic nephrolithiasis, membranous nephropathy, and acute tubular necrosis after coronary artery bypass grafting.
Thirty catheters had primary failure at the time of exteriorization (42.9%). Thirty-four obstructed catheters were located in the pelvis (49.2%), 21 in the lower abdomen (30.4%), and 14 in the upper abdomen (20.3%) (Table 3). An abdominal radiograph was not available for one patient.
Table 3.
Peritoneal dialysis catheter fluoroscopic manipulation results
| Result | Percentage (Ratio) |
|---|---|
| Overall success rate | 62.9 (44/70) |
| Success rate by position | |
| pelvis | 73.5 (25/34) |
| lower abdomen | 57.1 (12/21) |
| upper abdomen | 42.9 (6/14) |
| Success in primary failure | 46.7 (14/30) |
| Success in secondary failure | 75.0 (30/40) |
| Time gained after first successful manipulation (d), median (interquartile range) | 469 (171–856) |
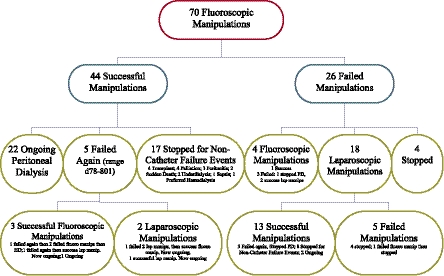
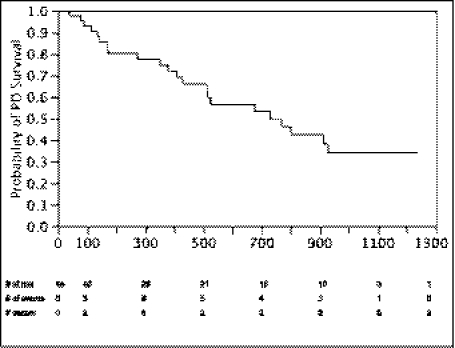
Of the 70 initial catheter manipulations, 44 were functional at 30 days (62.9%); 22 of these patients continue on PD at the present time, with the longest being 1237 days from manipulation to the end of the study. As shown in Figure 1, mechanical failure occurred again after the 30-day window in five catheters. Three of the mechanical failures were successfully treated again with fluoroscopic manipulation and two were treated with laparoscopic manipulations, one of which was successful. Seventeen of the patients with successful manipulations eventually discontinued PD for reasons unrelated to PD catheter mechanical failure. Of the 26 failed manipulations, 4 patients had repeat fluoroscopic manipulations (1 success; primary failure, lower abdomen), 18 patients had laparoscopic manipulations (13 successes; 6 primary failures and 7 secondary failures, 5 in the upper abdomen, 3 in the lower abdomen, 4 in the pelvis, and 1 unknown), and 4 patients were transferred immediately to hemodialysis. For the patients who failed a fluoroscopic manipulation and underwent a subsequent laparoscopic surgery, the PD catheters were found to be trapped in omentum (n=6), trapped in adhesions (n=3), trapped between loops of bowel (n=2), trapped in fat (n=1), blocked with fibrin or blood clots (n=4), and unclear cause of malfunction (n=2). Median time on PD gained by successful manipulation (including recurrent failures, stopping for reasons unrelated to catheter mechanical failure, and ongoing at the end of the study) after manipulation was 469 days (Figure 2).
Figure 1.
Flowchart of individual patient outcomes after initial fluoroscopic manipulation.
Figure 2.
Peritoneal dialysis survival after a first successful fluoroscopic manipulation.
Complications
Two of the initial manipulations were associated with the development of culture negative peritonitis 1 day after the procedure (2.9%). Both patients were treated successfully without further interruption to dialysis or need for catheter removal. No other complications were noted.
Predictors of Success
As shown in Table 3, in univariate analysis, catheters located in the pelvis were significantly more likely to have successful manipulations compared with those in the upper abdomen (73.5% versus 42.9%, P=0.01). There was no significant difference between catheters located in the pelvis relative to the lower abdomen (73.5% versus 57.1%, P=0.72). Catheters that were previously functional (secondary failure) were significantly more likely to be successfully manipulated than those that failed at exteriorization (primary failure) (75.0% versus 46.7%, P=0.04). Duration of time embedded, previous hemodialysis, and number of intra-abdominal surgeries were not correlated with likelihood of successful manipulation.
The results of the multivariate logistic regression model are shown in Table 4. Only pelvic location of catheter tip and secondary catheter failure were associated with increased odds of successful manipulation.
Table 4.
Logistic regression model of failure of fluoroscopic manipulation at 30 days
| Odds Ratio | P Value | |
|---|---|---|
| Univariate analysis | ||
| primary failure (at exteriorization) versus secondary failure (after functioning) | 2.80 (1.03–7.62) | 0.04 |
| length of time between insertion and first access (per 100 d) | 1.02 (0.88–1.09) | 0.72 |
| catheter position | ||
| pelvis | Reference | |
| lower abdomen | 2.78 (0.82–9.09) | 0.10 |
| upper abdomen | 7.14 (1.54–33.3) | 0.01 |
| hemodialysis versus no hemodialysis | 1.59 (0.49–5.26) | 0.45 |
| time on hemodialysis (per 10 d) | 1.09 (0.88–1.32) | 0.43 |
| previous intra-abdominal operations (n) | ||
| none | Reference | |
| 1–2 | 0.92 (0.33–2.56) | 0.87 |
| 3–4 | 0.24 (0.03–2.22) | 0.21 |
| Multivariate analysisa | ||
| primary (failed at exteriorization) versus secondary (failed after functioning) | 5.01 (1.47–17.12) | 0.01 |
| catheter position | ||
| pelvis | Reference | |
| lower abdomen | 1.00 (0.43–4.35) | 0.13 |
| upper abdomen | 12.5 (2.13–50.0) | 0.01 |
Only type of failure and position of catheter were retained for the multivariable analysis because P values were <0.20 in the univariate analysis.
Discussion
In our series of 70 PD patients with nonfunctional catheters, fluoroscopic manipulation was successful in 63% of patients. The two predictors of successful manipulation were pelvic location of the catheter tip and secondary failure. These findings are important because they help address some of the controversies around fluoroscopic manipulation by identifying a subgroup of patients with obstructed catheters that have a high rate of success with this procedure.
Previously reported success rates for fluoroscopic manipulation vary considerably from 25% to 67% (6–9). Our overall success rate is comparable with the upper end of these reported rates. When only catheters that were properly positioned in the pelvis and those that failed after a period of normal use (secondary failure) were considered, our overall success rate was quite high, in the range of 75%. Furthermore, our complication rate was very low, with peritonitis rates that were consistent with the background rate of PD peritonitis in our unit. Importantly, no complications required termination of PD, suggesting that there is little downside to a strategy of fluoroscopic manipulation for obstructed catheters.
The lower rate (approximately 45%) of successful manipulation for catheters migrated to the upper abdomen and those with primary failure suggest that fluoroscopic manipulation cannot always correct significant anatomic abnormalities such as omental wrapping of the catheter, compartmentalization by adhesions in the lesser sac, or excessive torque on the catheter due to poor surgical technique. These findings are more likely to cause both primary failure as well as migration to the upper abdomen. Our findings for patients who underwent a surgical laparoscopy after a failed fluoroscopic manipulation support this because the majority of catheters were trapped in adhesions, omentum, loops of bowel, or fat. Secondary obstruction of the catheter without migration out of the pelvis may be due to fibrin obstruction of the lumen, which can be cleared by a guidewire. The only inflow obstruction and the majority of catheters with both inflow and outflow obstruction were in a pelvic location supporting the possibility of a complete or partial intraluminal obstruction that may have been cleared by the guidewire. Secondary mechanical failure with malposition may be related to the development of fine pelvic adhesions that can be easily broken by movement of the catheter with a guidewire. Nevertheless, a success rate of 45% for primary failure and migrated catheters still suggests that fluoroscopic manipulation is worthwhile before contemplating a laparoscopic manipulation.
The reported success with laparoscopic manipulation is higher than with fluoroscopic manipulation (93%–100%), but this is accompanied by a higher complication rate (7%–39%). The documented complications include peritonitis, tunnel infections, dialysate leaks, hernias, and hemorrhages (10–14). In addition, due to the peritoneal punctures, PD may have to be discontinued for a few weeks after laparoscopic surgery and this often requires a temporary transition to hemodialysis while the incisions heal. In comparison, no patients had to be transitioned to hemodialysis due to complications of fluoroscopic manipulation, and our success rate was 63% overall. For this reason, we believe that fluoroscopic manipulation should be attempted first in the majority of patients because this method provides a safe and less expensive alternative to laparoscopic manipulation and is successful in the majority of patients.
Our study has a number of limitations. Our single-center experience may not be representative of the more global community of PD patients with catheter malfunction due to issues of patient selection, surgical experience, and skill of our interventional radiologists. Our use of prophylactic antibiotics may have prevented episodes of peritonitis. We were unable to determine specific causes of catheter malfunction because this is seldom determined with certainty during a fluoroscopic manipulation. We retained all three abdominal catheter positions in our multivariable analysis in spite of the small number of patients per group, which may have affected our estimated odds ratios. Lastly, in our retrospective study, we only included patients who underwent fluoroscopic manipulation, leading to a potential overestimation of the success rate because we excluded the potentially more severe patients who were sent directly for laparoscopic manipulation.
Further opportunities for research include determining if there are other variables that are important for successful fluoroscopic manipulation such as the appearance of the abdomen at the time of catheter insertion, the insertion technique, and the expertise of the radiologist involved. Ideally, a randomized controlled trial to compare the results of fluoroscopic versus laparoscopic manipulation of failed PD catheters would be beneficial in defining the optimal technique with respect to success rates, cost, and patient acceptance. In conclusion, our results validate fluoroscopic manipulation as a useful tool for correcting failed PD catheters. Until further studies are available, fluoroscopic manipulation should be considered the first choice for patients with refractory PD catheter obstruction because of the relatively high success rate and low complication rate.
Disclosures
None.
Acknowledgments
D.Z. receives salary support from the Department of Medicine at the Ottawa Hospital.
This study was presented at the World Congress of Nephrology, April 8–12, 2011, in Vancouver, British Columbia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Mujais S, Story K: Peritoneal dialysis in the US: Evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 103: S21–S26, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Shahbazi N, McCormick BB: Peritoneal dialysis catheter insertion strategies and maintenance of catheter function. Semin Nephrol 31: 138–151, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Crabtree JH: Fluoroscopic placement of peritoneal dialysis catheters: A harvest of the low-hanging fruits. Perit Dial Int 28: 134–137, 2008 [PubMed] [Google Scholar]
- 4.McCormick BB, Brown PA, Knoll G, Yelle JD, Page D, Biyani M, Lavoie S: Use of the embedded peritoneal dialysis catheter: Experience and results from a North American Center. Kidney Int Suppl 70: S38–S43, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brown PA, McCormick BB, Knoll G, Su Y, Doucette S, Fergusson D, Lavoie S: Complications and catheter survival with prolonged embedding of peritoneal dialysis catheters. Nephrol Dial Transplant 23: 2299–2303, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kappel JE, Ferguson GMC, Kudel RM, Kudel TA, Lawlor BJ, Pylypchuk GB: Stiff wire manipulation of peritoneal dialysis catheters. Adv Perit Dial 11: 202–207, 1995 [PubMed] [Google Scholar]
- 7.Savader SJ, Lund G, Scheel PJ, Prescott C, Feeley N, Singh H, Osterman FA, Jr: Guide wire directed manipulation of malfunctioning peritoneal dialysis catheters: A critical analysis. J Vasc Interv Radiol 8: 957–963, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Simons ME, Pron G, Voros M, Vanderburgh LC, Rao PS, Oreopoulos DG: Fluoroscopically-guided manipulation of malfunctioning peritoneal dialysis catheters. Perit Dial Int 19: 544–549, 1999 [PubMed] [Google Scholar]
- 9.Lee CM, Ko SF, Chen HC, Leung TK: Double guidewire method: A novel technique for correction of migrated Tenckhoff peritoneal dialysis catheter. Perit Dial Int 23: 587–590, 2003 [PubMed] [Google Scholar]
- 10.Brandt CP, Ricanati ES: Use of laparoscopy in the management of malfunctioning peritoneal dialysis catheters. Adv Perit Dial 12: 223–226, 1996 [PubMed] [Google Scholar]
- 11.Amerling R, Maele DV, Spivak H, Lo AY, White P, Beaton H, Rudick J: Laparoscopic salvage of malfunctioning peritoneal catheters. Surg Endosc 11: 249–252, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Yilmazlar T, Yavuz M, Ceylan H: Laparoscopic management of malfunctioning peritoneal dialysis catheters. Surg Endosc 15: 820–822, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Donovan JF: Laparoscopic omentectomy for salvage of peritoneal dialysis catheters. J Endourol 16: 241–244, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Yilmazlar T, Kirdak T, Bilgin S, Yavuz M, Yurtkuran M: Laparoscopic findings of peritoneal dialysis catheter malfunction and management outcomes. Perit Dial Int 26: 374–379, 2006 [PubMed] [Google Scholar]