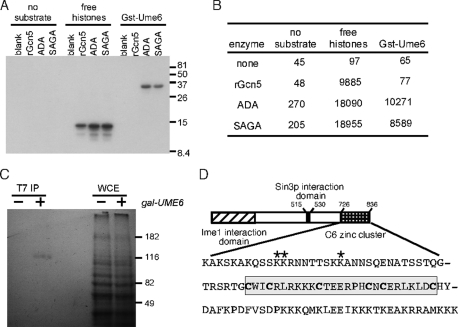
FIGURE 1:
Ume6p is a substrate of acetyltransferase complexes. (A) Affinity-purified acetyltransferase fractions or recombinant Gcn5p were incubated with GST-Ume6721-831 or the four histones. The reactions were split, with one-half separated by PAGE and fluorographed or quantitated (B) by liquid scintillation spectroscopy (dpm). (C) Wild-type strain RSY10 harboring GAL-T7-UME6 expression plasmid (+) or vector control (–) was grown in the presence of 14C-acetyl-CoA. Extracts derived from these cultures were immunoprecipitated with T7 mAb, and the immunoprecipitates were separated by PAGE and then visualized by fluorography. Whole-cell extracts (WCEs) controlled for equal protein loading and labeling. (D) Acetylation occurs in a region adjacent to the C6 zinc-cluster DNA-binding domain of Ume6p. The sequence of the last 110 amino acids of Ume6p (726–836) is presented. The acetylated residues are indicated by the asterisks. The cysteines making up the C6 zinc cluster are in boldface and boxed. The Ime1p and Sin3p interaction domains are indicated.