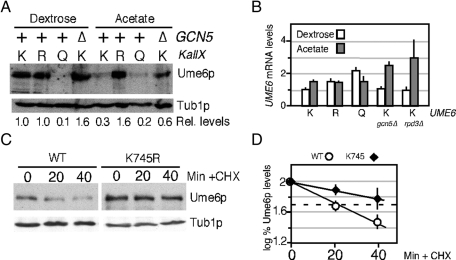
FIGURE 3:
Acetylation promotes Ume6p destruction. (A) Steady-state levels of endogenous Ume6p (RSY1079), Ume6pKALLR (RSY1149), Ume6pKALLQ (RSY1226), or Ume6p in a gcn5∆ mutant (∆, RSY1091) were determined by Western blot analysis of extracts derived from log-phase dextrose or acetate cultures as indicated. The blots were stripped and reprobed for tubulin to calculate relative levels of the Ume6p derivatives. UME6 alleles: K, wild type; R, KallR; Q, KallQ. (B) Quantitative PCR was used to monitor UME6 mRNA in strains containing the chromosomal wild-type (K), KallR (R), and KallQ (Q) alleles grown to mid-log phase in dextrose or acetate medium as indicated. Experiments conducted in gcn5∆ or rpd3∆ mutants with wild-type UME6 are indicated. All values were normalized to internal ENO1 mRNA concentrations with UME6 levels in dextrose medium set at 1. Error bars indicate SEM from triplicate replicates from two independent cultures. (C) Half-life determinations of Ume6p and Ume6pK745R. Extracts prepared from G2-arrested (0 min) and released cultures were probed for Ume6p or Ume6pK745R. See Materials and Methods for details. Tub1p levels served as a loading control. (D) Quantification of the results indicated in C are shown (see Materials and Methods for details). Half-lives were determined by linear regression analysis (linear correlation, >0.9) for two independent experiments. Dashed line indicates 50% initial protein levels.