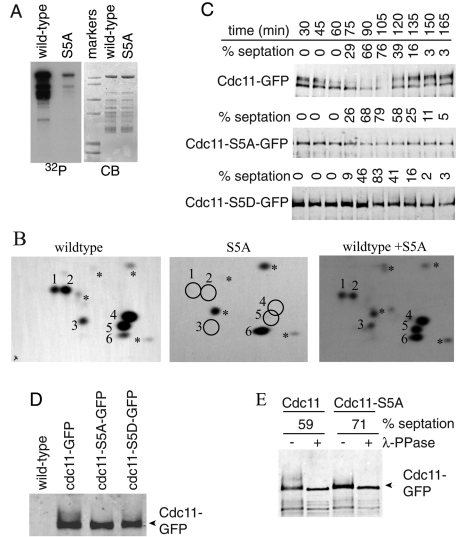
FIGURE 2:
Identification of Sid2-mediated Cdc11 phosphorylation sites. (A) Sid2-Myc13 was immunoprecipitated from cdc16-116 cells that had been shifted to 36ºC for 2.5 h and incubated with MBP-Cdc11-(1-660) (wild-type) or MBP-Cdc11-S5A. (B) Phosphotryptic peptides from the indicated Cdc11 proteins were separated in two dimensions with the anode on the left. The positions of major phosphopeptides are numbered. The asterisks indicate minor phosphopeptides. Equal counts of wild-type MBP-Cdc11-(1-660) or the S5A mutant were analyzed individually (left two panels) or mixed together (right panel) to prove the correct assignment of phosphorylation sites. (C) Sid2 phosphorylation sites contribute to Cdc11 mitotic phosphorylation. cdc11-GFP, cdc11-S5A-GFP, and cdc11-S5D-GFP strains were synchronized in G2 phase using temperature shift of cdc25-22 to 36ºC for 4 h, and samples were taken every 15 min after return to 25ºC. Cdc11 proteins were isolated from cell pellets by immunoprecipitation and detected by immunoblotting with anti-GFP antibody. Cell cycle progression was monitored by formation of septa. (D) Samples from the 60-min time point in (C) were run on the same gel to demonstrate comigration of the most rapidly migrating Cdc11 band. (E) An anti-GFP immunoprecipitate of wild-type Cdc11 or Cdc11-S5A isolated from a similar cdc25 block-and-release experiment at the indicated septation indices was treated (+) or not (−) with λ-phosphatase before immunoblotting.